Preparation method of moxidectin impurities
A technology for moxidectin and impurities, applied in the field of drug synthesis, can solve problems such as no reports yet, and achieve the effects of reasonable route design, strong reaction controllability and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A preparation method for moxidectin impurities, comprising the following steps:
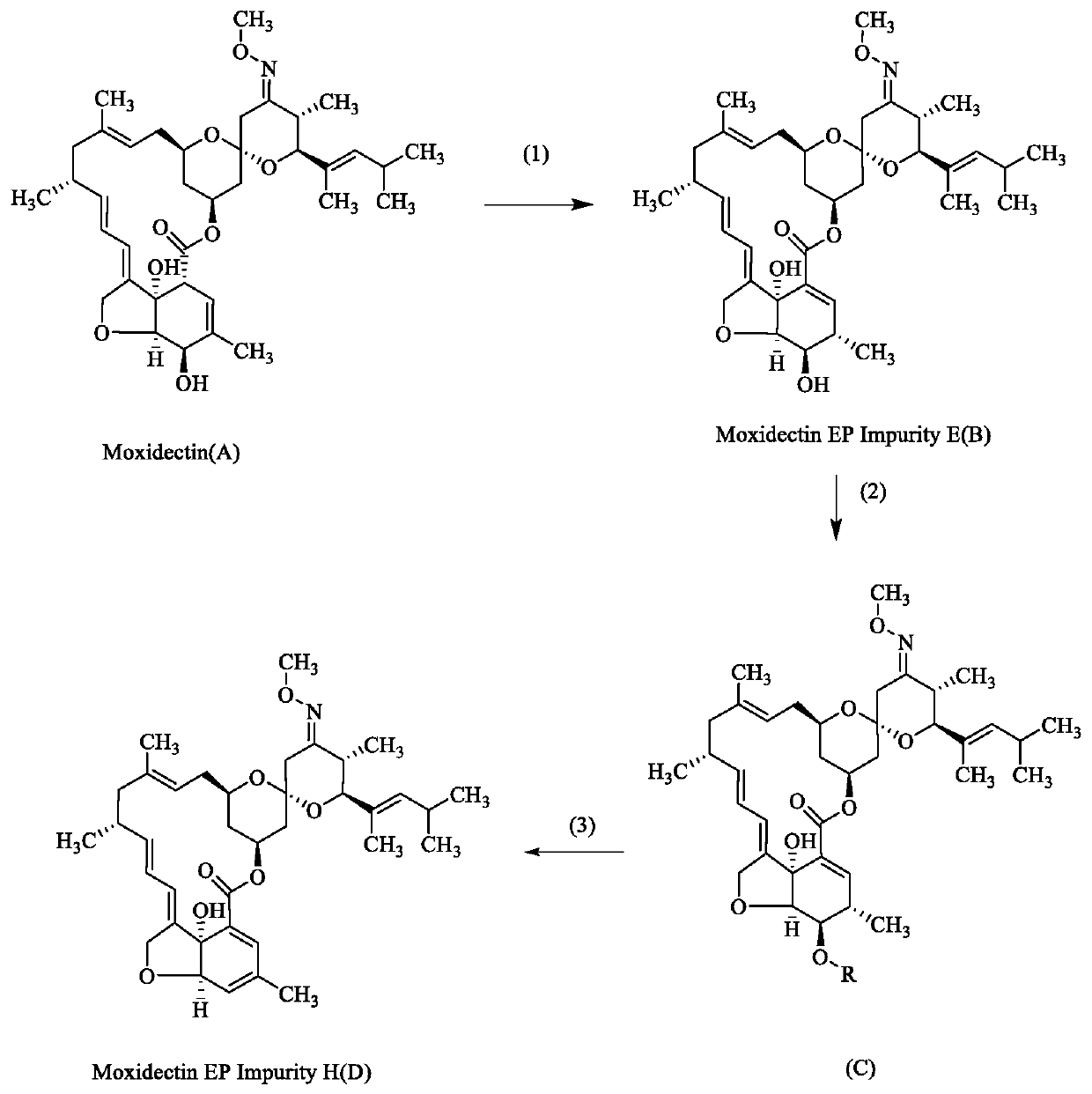
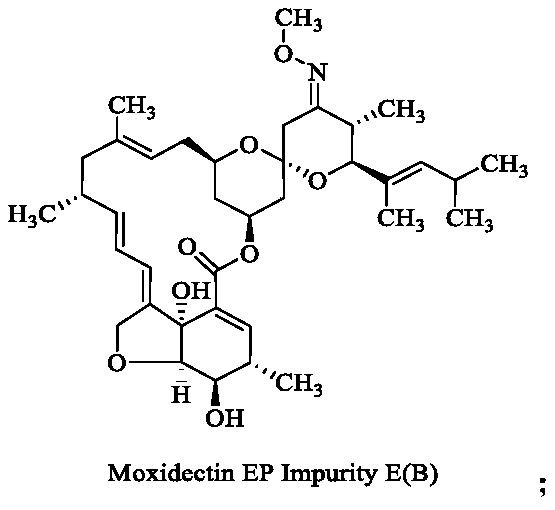
[0021] (1) Dissolve 5 g of moxidectin in 30 mL of tetrahydrofuran, add 3 g of potassium carbonate, and react the mixture at 100° C. for 1 hour. Thin-layer chromatography shows that the reaction is complete. Concentrate the reaction solution to remove tetrahydrofuran, and purify on a silica gel column to obtain 4.5 g of compound B, yield 90%; MS: 662.3[M+Na]; 1H NMR (400MHz, CDCl 3 ):δ0.72(dd,1H),0.91(d,3H),0.97(d,3H),0.99(d,3H),1.05(d,3H),1.22(d,3H),1.46(s, 3H), 1.63(s, 3H), 1.8(m, 3H), 1.9(d, 1H), 2.07(dd, 1H), 2.2(m, 4H), 2.3~2.4(m, 2H), 2.5~2.7 (m,2H),3.28(d,1H),3.5~3.7(m,3H),3.84(s,3H),4.05(d,1H),4.53(dd,2H),4.79(s,1H), 4.90(t,1H),5.17(d,1H),5.34(dd,2H),5.70(dd,1H),6.10(dt,1H),6.15(s,1H);
[0022] (2) Dissolve 4g of compound B in 70mL of dichloromethane, add 3g of triethylamine and 3.2g of benzoic anhydride at room temperature, react at 25°C for 2 hours, thin layer chromatography sh...
Embodiment 2
[0025] A preparation method for moxidectin impurities, comprising the following steps:
[0026] (1) Dissolve 15.0 g of moxidectin in 30 mL of tetrahydrofuran, add 6.0 g of triethylamine, and react the mixture at 100° C. for 2 hours. Thin layer chromatography shows that the reaction is complete. Purified to obtain 14.2g of compound B, yield 93.3%;
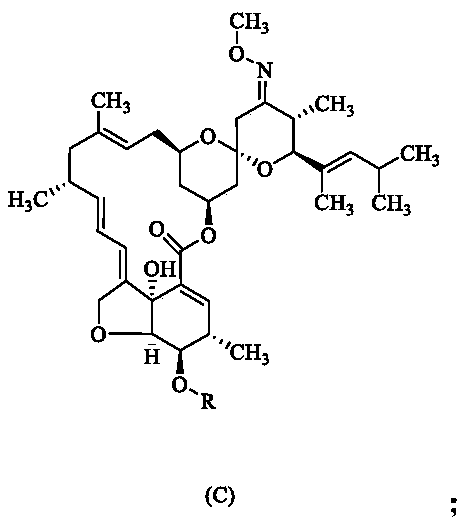
[0027] (2) Dissolve 10 g of compound B in 70 mL of dichloromethane, add 3 g of triethylamine and 4.5 g of methanesulfonyl chloride at room temperature, and react at 40 ° C for 2 hours. Thin layer chromatography shows that the reaction is complete, and water is added to separate the organic After phase, silica gel column purification to obtain 9.5g of compound C, yield 89.20%;
[0028] (3) Dissolve 5.2g of compound C in acetonitrile, add 4.1g of potassium carbonate at room temperature and react at 40°C for 2 hours, thin-layer chromatography shows that the reaction is complete, after concentration to remove tetrahydrofuran, silica ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com