Preparation method of deuterated telaprevir
A technology of telaprevir and telaprevir, which is applied in the field of preparation of deuterated telaprevir, can solve problems such as unreported preparation methods of deuterated telaprevir, and achieves the effects of reasonable route design and simple and convenient preparation steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
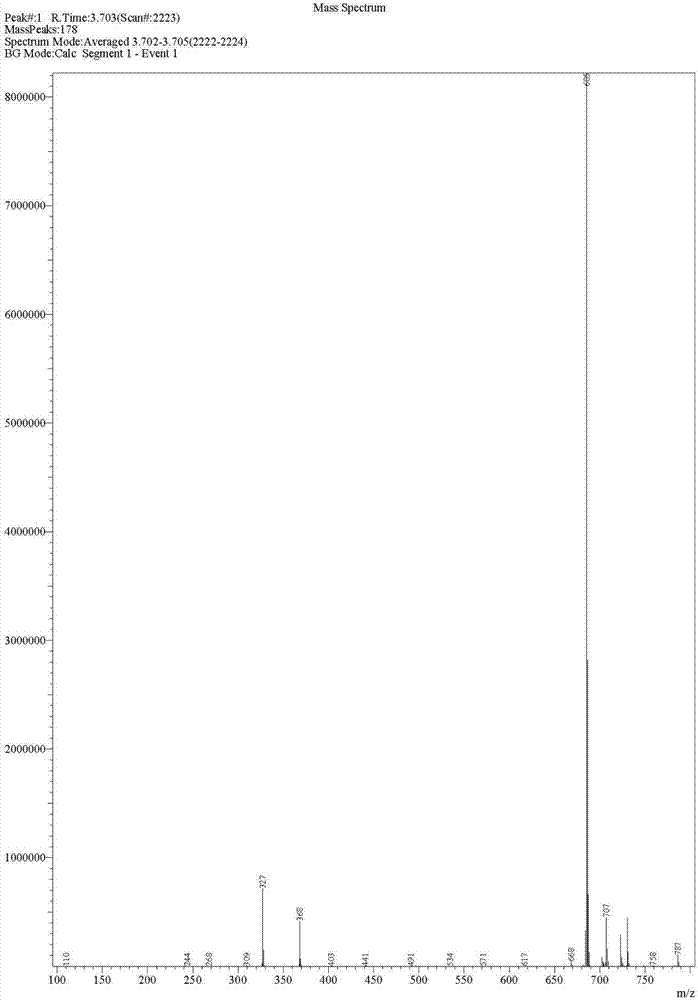
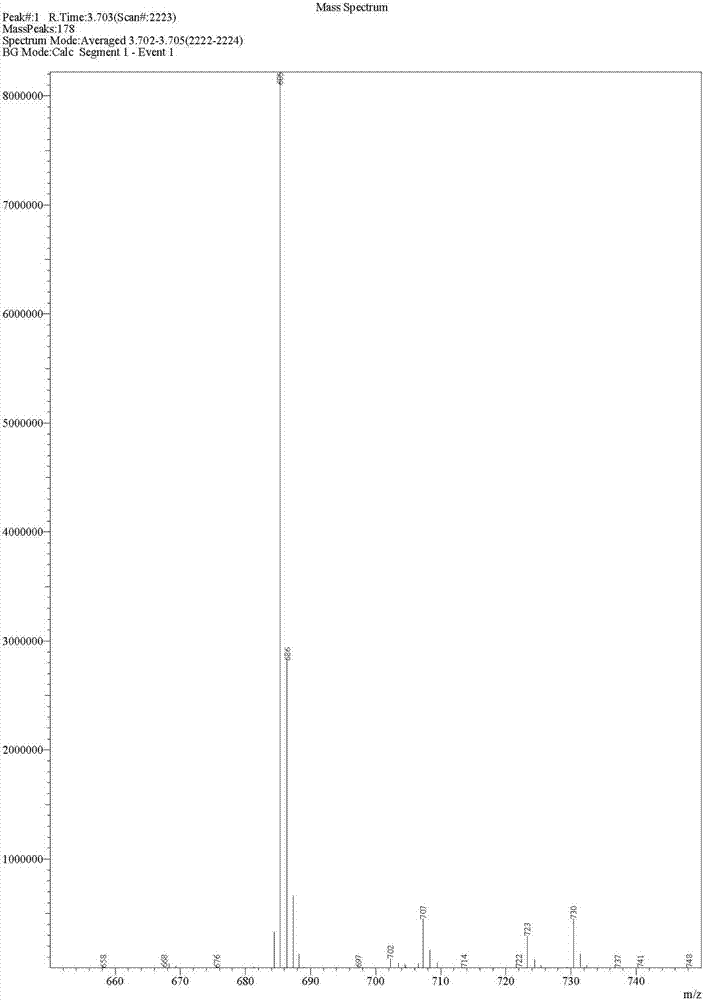
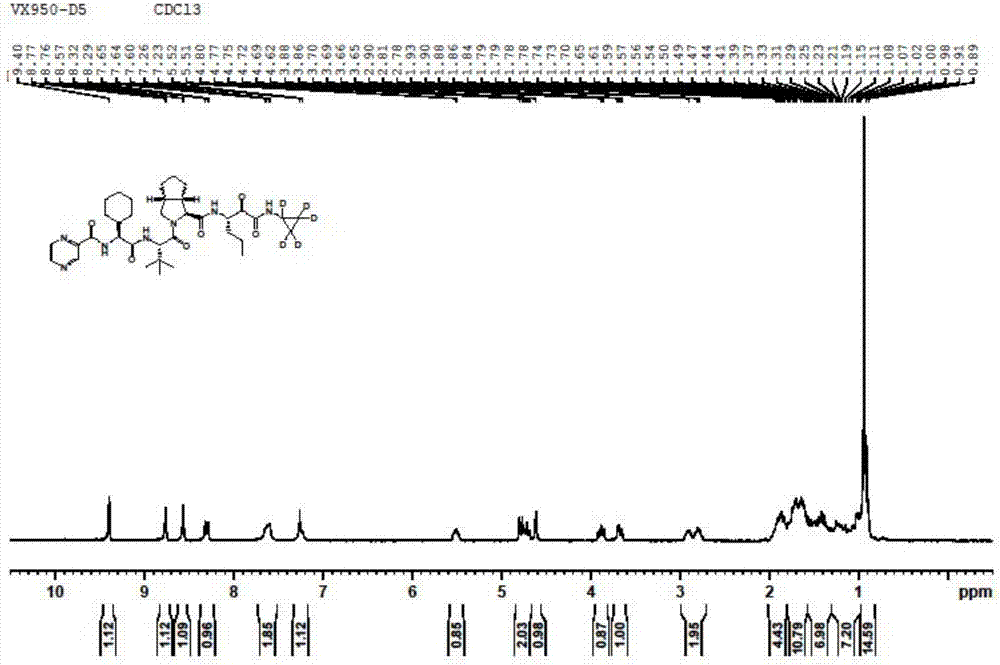
Image
Examples
Embodiment 1
[0032] Embodiment 1 Preparation of the deuterated compound shown in formula (VII)
[0033] The synthesis process is as follows:
[0034]
[0035] Add 30mL of methanol to the reaction flask, add 5.5g of sodium metal in batches, stir until the sodium block disappears to obtain sodium methylate (NaOMe), then add 28g of hexadeuterio ethyl 4-chlorobutyrate shown in formula (II) , heated and refluxed for 12 hours, lowered to 20-30 ° C, added 50 g of 30% sodium hydroxide aqueous solution, reacted for 6 hours, concentrated, adjusted pH to 3 with concentrated hydrochloric acid, extracted twice with dichloromethane, concentrated to dryness, and obtained yellow Liquid 13.5g, yield 83%. Add 3.6 g of the above yellow liquid, 10 mL of benzyl alcohol (PhCH 2 OH), 40mL of toluene, 13g of diphenylphosphoryl azide (DPPA) and 4.8g of triethylamine (TEA), heated to reflux for 5 hours, cooled to 20-30°C, and the reaction solution was sequentially filled with 1mol / L (1N) After washing with hy...
Embodiment 2
[0037] The synthesis process is as follows:
[0038]
[0039] (1) Preparation of an organic phase comprising a deuterated compound as shown in formula (IX)
[0040] Add 210mL of dichloromethane to the reaction flask, cool down to 0~5°C, add 35g telaprevir intermediate shown in formula (VIII), 8.8g 4-hydroxybenzotriazole (HBOt), 12.4g 1 -(3-Dimethylaminopropyl)-3-ethylcarbimide (EDCI), stir and mix, then add 14.4g of the deuterated compound shown in formula (VII), slowly add 12.9mL of N- Methylmorpholine was naturally heated to 20-30°C for 8 hours, and the reaction liquid was washed with water, 1N hydrochloric acid, and 5% sodium bicarbonate in sequence, and the organic phase was directly put into the next reaction after liquid separation.
[0041] (2) preparation of deuterated telaprevir as shown in formula (I)
[0042] Add 0.75g tetramethylpiperidine oxide (TEMPO) and 120g 7% sodium bicarbonate aqueous solution into the organic phase prepared in Example 2, cool to 0-5°C,...
Embodiment 3
[0044] (1) Preparation of an organic phase comprising a deuterated compound as shown in formula (IX)
[0045] Add 210mL of dichloromethane to the reaction flask, cool down to 0-5°C, add 31.5g telaprevir intermediate shown in formula (VIII), 6.3g 4-hydroxybenzotriazole (HBOt), 10.5g 1-(3-Dimethylaminopropyl)-3-ethylcarboimide (EDCI), stirred and mixed, then added 12.2g of the deuterated compound shown in formula (VII), slowly added dropwise 12.9mL N -Methylmorpholine, naturally heated to 20-30°C for 6 hours, the reaction solution was washed with water, 1N hydrochloric acid, and 5% sodium bicarbonate in sequence, and the organic phase was directly put into the next reaction after liquid separation.
[0046] (2) preparation of deuterated telaprevir as shown in formula (I)
[0047] Add 0.75g tetramethylpiperidine oxide (TEMPO) and 120g 7% sodium bicarbonate aqueous solution into the organic phase prepared in Example 2, cool to 0-5°C, slowly add 70g 10-13% sodium hypochlorite aque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com