Preparation method of fluvastatin sodium dehydrated metabolite
A technology of fluvastatin sodium and metabolites, which is applied in the field of preparation of dehydrated metabolites of fluvastatin sodium, can solve problems such as tissue damage and gene changes, and achieve the effects of reasonable route design, high yield and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
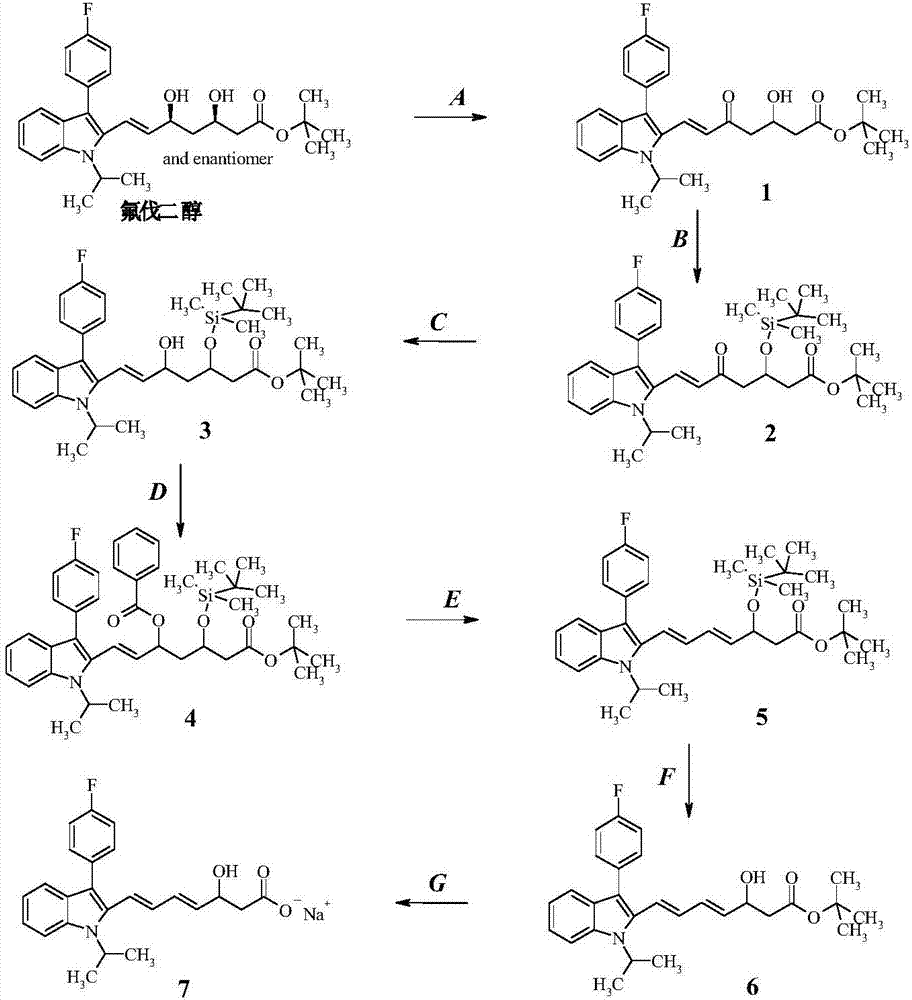
[0043] Such as figure 1 Shown, a kind of preparation method of fluvastatin sodium dehydration metabolite, it comprises the following steps:
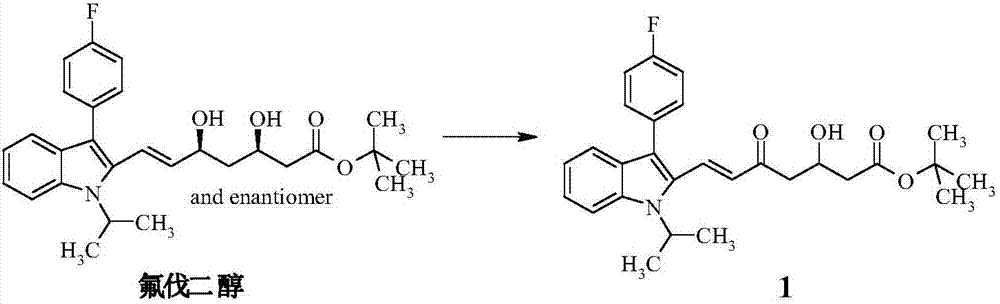
[0044] Step (A): Preparation of intermediate product (1)
[0045] 20g fluvadiol was dissolved in 400mL THF, added 11.1g manganese dioxide, stirred at room temperature after 12 hours, the reaction was complete, and the manganese dioxide was removed by suction filtration, the solid was washed with THF, the filtrate was concentrated, and purified with a chromatographic column to obtain the compound (1 ), yield 85.33%.
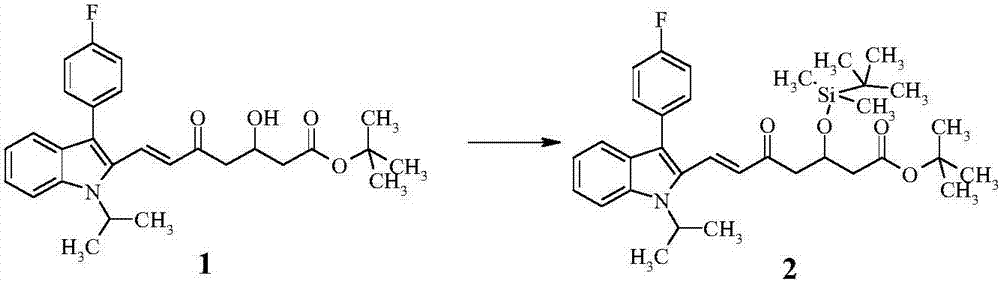
[0046] Step (B): Preparation of intermediate product (2)
[0047] Take 12g of compound (1) and dissolve it in 120mL of dichloromethane, add 5.3g of imidazole and 9.7g of tert-butyldimethylsilyl chloride, react at room temperature for 18 hours, TLC shows that the reaction is complete, the reaction solution is followed by water, 0.5N hydrochloric acid and chloride After washing with aqueous sodium solution, drying over anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com