Crystal material, preparation method and application thereof, positive electrode material of potassium ion battery and potassium ion battery comprising positive electrode material
A technology of crystalline materials and battery positive electrodes, which is applied in the direction of battery electrodes, secondary batteries, carboxylate preparation, etc., can solve the problems of high cost, complicated preparation process, and unsatisfactory performance, and achieve fewer steps, simple process, and good battery life. The effect of chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] In the second aspect, in at least one embodiment, a method for preparing the above-mentioned crystal material is provided, comprising the following steps: uniformly mixing the potassium source, the M source, the oxalate source and a solvent, and then performing a solvothermal reaction to obtain the crystal material.
[0072] The above preparation method has simple process, few steps, convenient operation, low cost and is suitable for industrial production. Meanwhile, the prepared crystal material has stable structure, good electrochemical performance, high discharge voltage and high charge and discharge capacity.
[0073] Preferably, the molar ratio of M source, oxalate source, potassium source and solvent is 1:(1-3):(10-40):(100-400). The molar ratios above are typically but not limited to 1:1:10:100, 1:2:10:100, 1:3:10:100, 1:1:20:100, 1:1:30:100, 1:1:35:100, 1:1:40:100, 1:2:20:100, 1:2:30:100, 1:2:35:100, 1:2:40:100, 1: 3:20:100, 1:3:30:100, 1:3:35:100, 1:3:40:100, ...
Embodiment 1
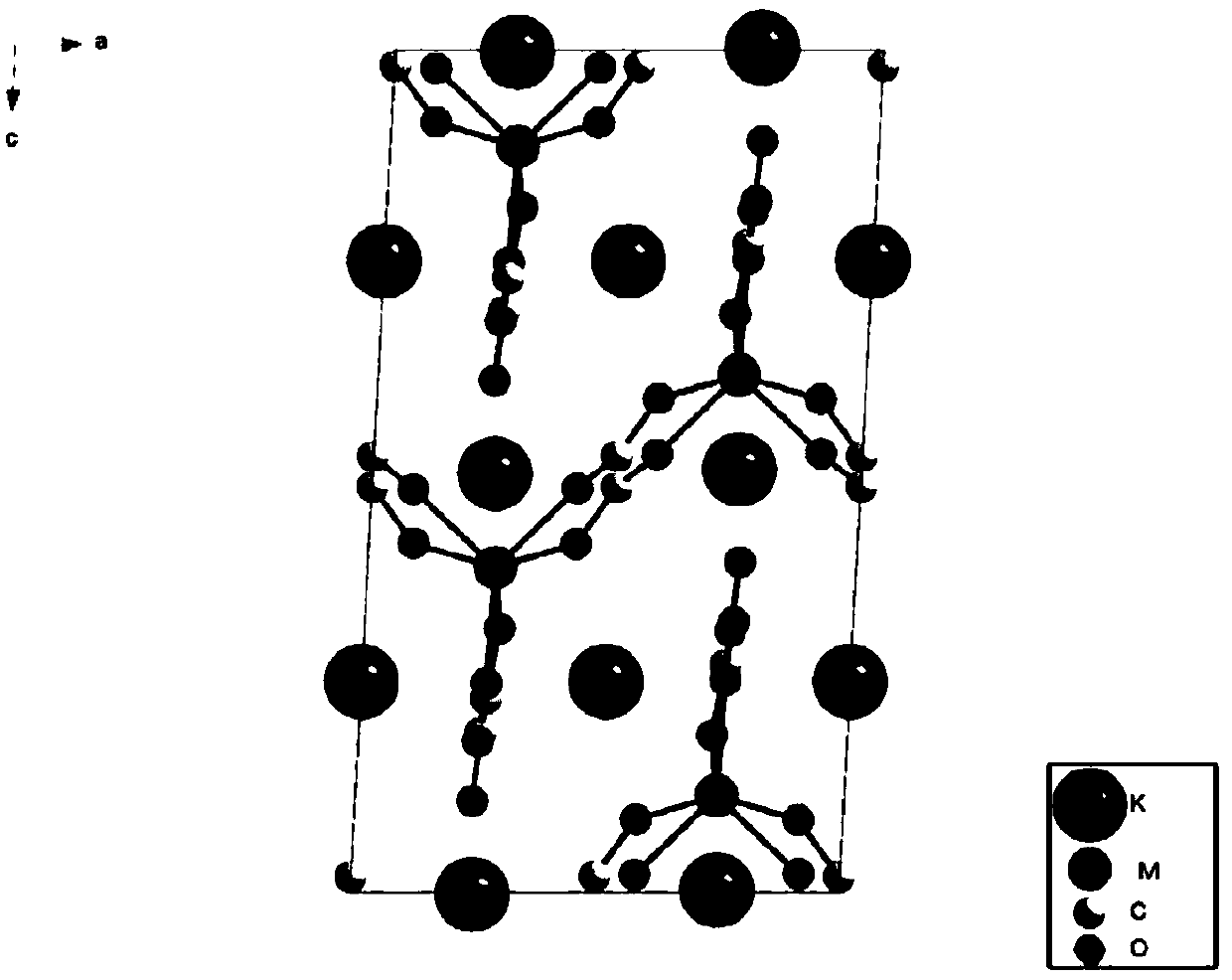
[0200] A kind of crystalline material, its preparation method comprises: MnCl 2 0.6113g, K 2 CO 3 1.3425g, H 2 C 2 o 4 2(H 2 O) 1.347g, KCl 9.053g, H 2 O 17.5g was added to a 50mL hydrothermal synthesis reactor; the reactor containing the raw materials was heated at a constant temperature of 200°C for 48h and then cooled to room temperature to obtain K 2 Mn(C 2 o 4 ) 2 Crude product; take out the solid product in the reaction kettle and rinse it with absolute ethanol; add the rinsed solid product to 80 mL of glycerol, heat at 90°C for 6 hours to remove KCl impurities; filter and wash off the residual acrylic acid with absolute ethanol triol, dry to obtain the pure phase of K 2 Mn(C 2 o 4 ) 2 crystal particles.
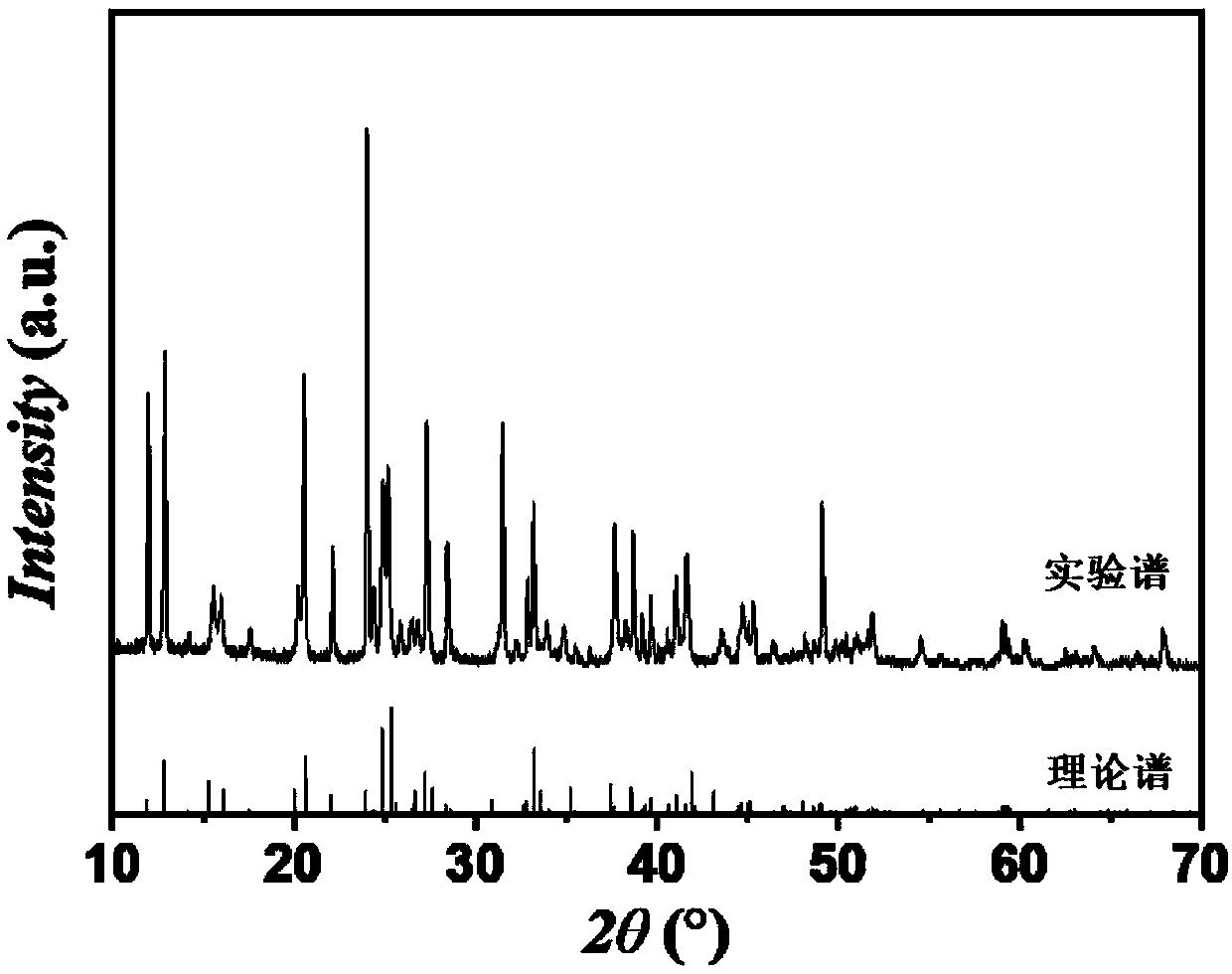
[0201] like image 3 Shown is the XRD (X-ray diffraction, X-ray diffraction) collection of spectra of the crystalline material that embodiment 1 prepares, as can be seen from the collection of spectra, this crystalline material is the K of pure phase ...
Embodiment 14
[0209] A kind of potassium ion battery, adopts following method to prepare:
[0210] (a) Preparing the negative electrode of the battery: Roll metal potassium on aluminum foil (i.e., the negative electrode current collector) to form a foil, and cut the obtained potassium-aluminum composite foil into discs with a diameter of 12mm, and use it as the negative electrode of the battery;
[0211] (b) Prepare the diaphragm: cut the glass fiber film into discs with a diameter of 16mm and use it as a diaphragm for subsequent use;
[0212] (c) Preparation of electrolyte: Weigh 1.410g of potassium hexafluorophosphate and add it to a mixed solvent of 4.962g of ethylene carbonate and 3.874g of dimethyl carbonate, stir until potassium hexafluorophosphate is completely dissolved, stir well and use as electrolyte spare;
[0213] (d) Preparation of battery positive electrode: 0.8g K 2 Mn(C 2 o 4 ) 2 Crystal powder, 0.1g carbon black, and 0.1g polyvinylidene fluoride were added to 2ml nitr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com