Immobilized whole-cells, preparation method of immobilized whole-cells, and application in production of 3-aminopropionic acid
A whole cell and cell technology, applied in the direction of immobilization on or in inorganic carrier, immobilization on/in organic carrier, biochemical equipment and methods, etc., can solve the problem of low bacterial tolerance, low yield, etc. problem, to achieve the effect of improving catalytic activity, good biocompatibility, and strong designability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 produces the construction of L-aspartic acid decarboxylase bacterial strain and cultivation process [1~3]
[0038] 1.1 Construction of strains
[0039] Extract the genomes of C.glutamicum, E.coli MG1655 and B.subtilis, use these three genomes as templates, and amplify the ADC gene panD by PCR (see Table 1). I double digestion, and then use T4 DNA ligase to connect to the pET28a vector that has also undergone double digestion, construct the recombinant plasmid pET28a-panDC.g, transform it into E.coli BL21 (DE3), and screen a single colony on the Kanna plate .
[0040] Table 1
[0041]
[0042] 1.2 Medium
[0043] Plate medium (L -1 ): peptone 10g, yeast powder 5g, sodium chloride 5g, agar 20g, kanamycin 0.05g, solvent is water.
[0044] LB medium (L -1 ): peptone 10g, yeast powder 5g, sodium chloride 5g, kanamycin 0.05g, and the solvent is water.
[0045] Sterilization method: the antibiotics were sterilized by filtration, and the rest were steril...
Embodiment 2
[0055] Example 2 Preparation process of immobilized whole cells
[0056] 2.1 Construction of strains
[0057] Refer to the construction of 1.1 bacterial strain in embodiment 1
[0058] 2.2 Medium
[0059] Refer to 1.2 medium in embodiment 1
[0060] 2.3 Plate culture
[0061] Refer to 1.3 plate culture in embodiment 1
[0062] 2.4 Seed liquid culture
[0063] Refer to 1.4 seed liquid culture in embodiment 1
[0064] 2.5 Shake flask fermentation culture
[0065] Refer to 1.5 shake flask fermentation culture in embodiment 1
[0066] 2.6 Preparation of immobilized whole cells
[0067] Resuspend the collected bacteria sludge with water to obtain a cell suspension with a concentration of OD600=15, mix 10 mL of a cell suspension with a concentration of OD600=15 with 5 mL of 45 mmol / L cobalt ion solution for 15 minutes, then add 5 mL of 80 mmol / L The dimethylimidazole solution was stirred at room temperature at a speed of 500 rpm for 30 to 60 minutes, and then washed with wa...
Embodiment 3
[0068] Embodiment 3 analytical method
[0069] 3.1 Catalyzed method for producing 3-aminopropionic acid
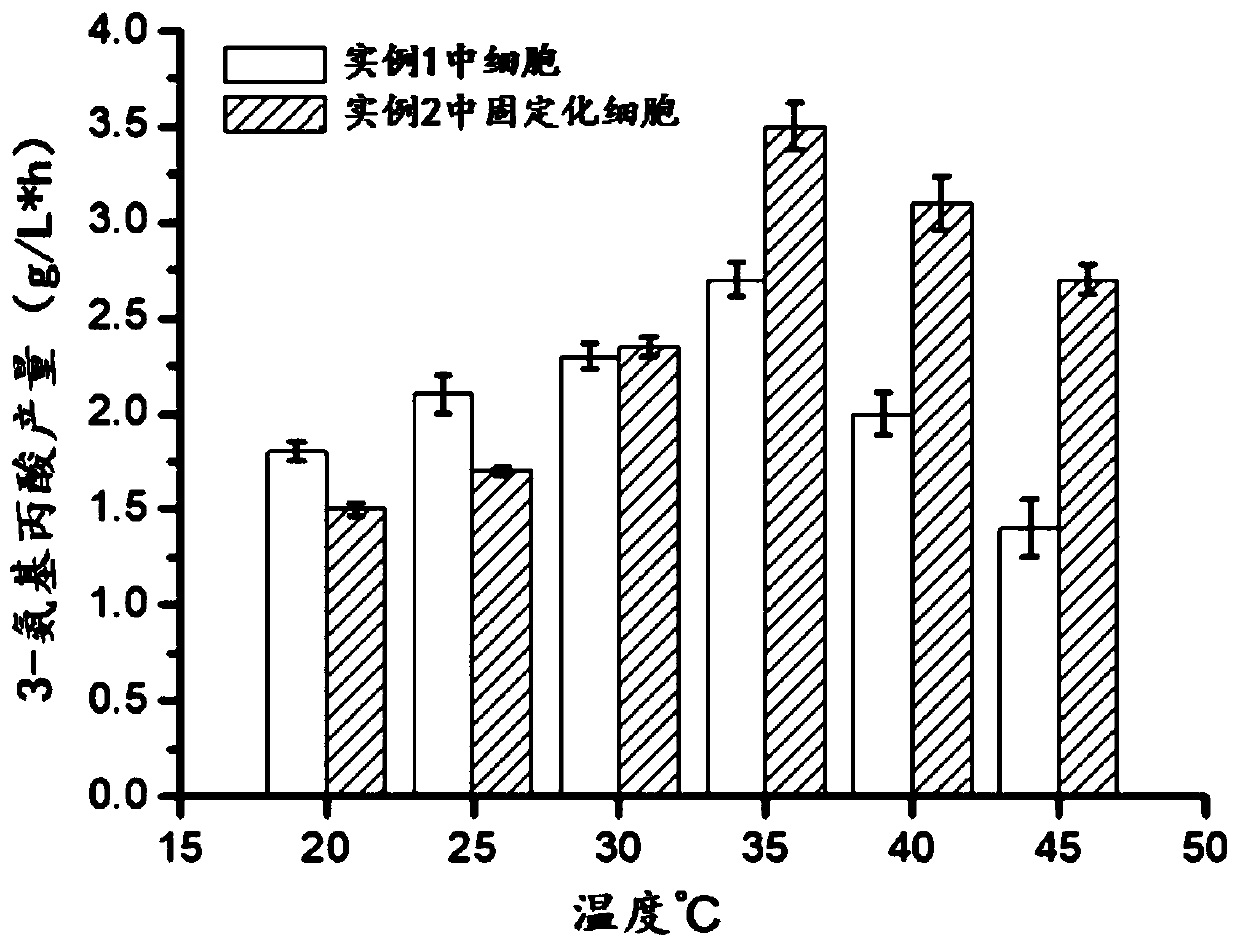
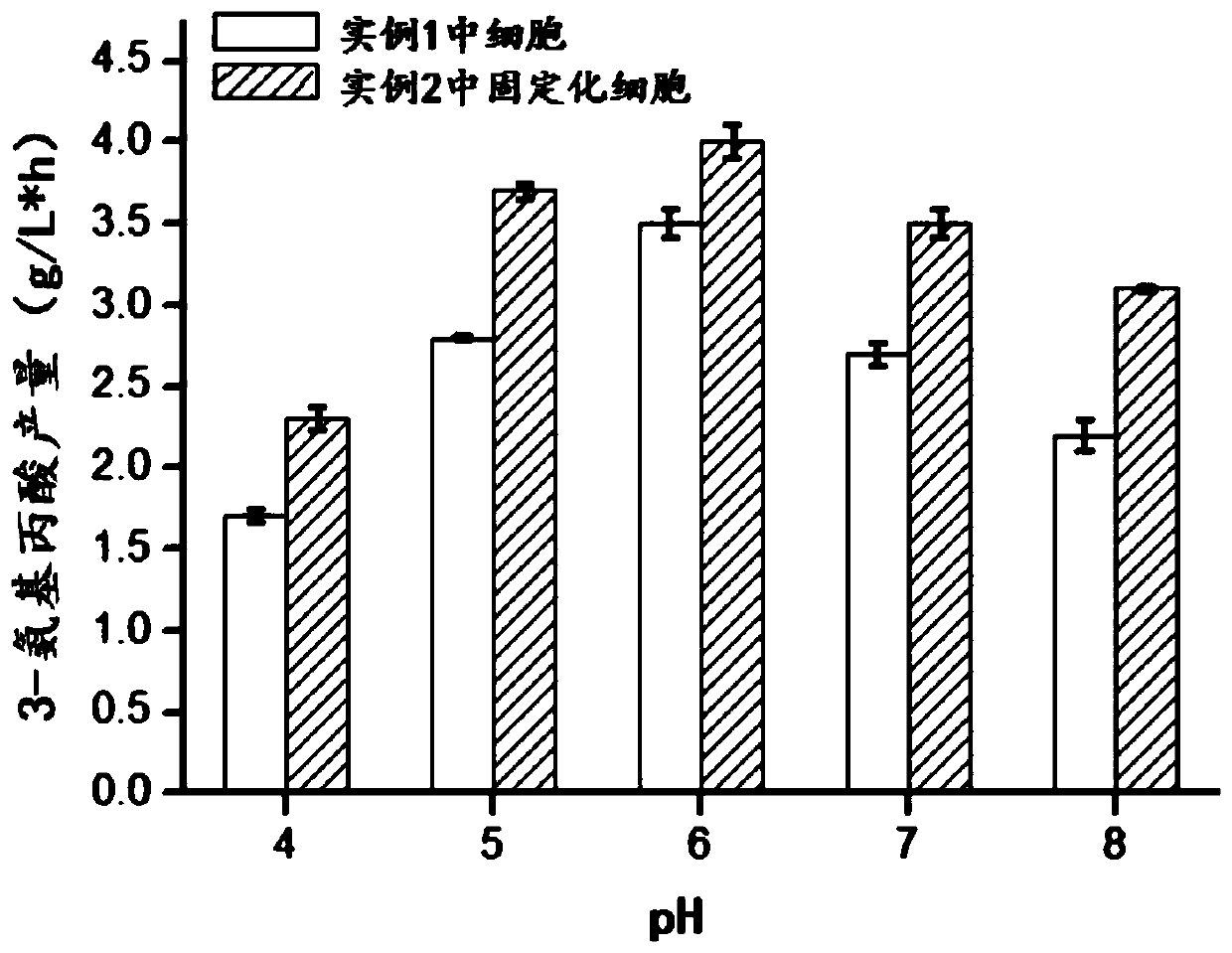
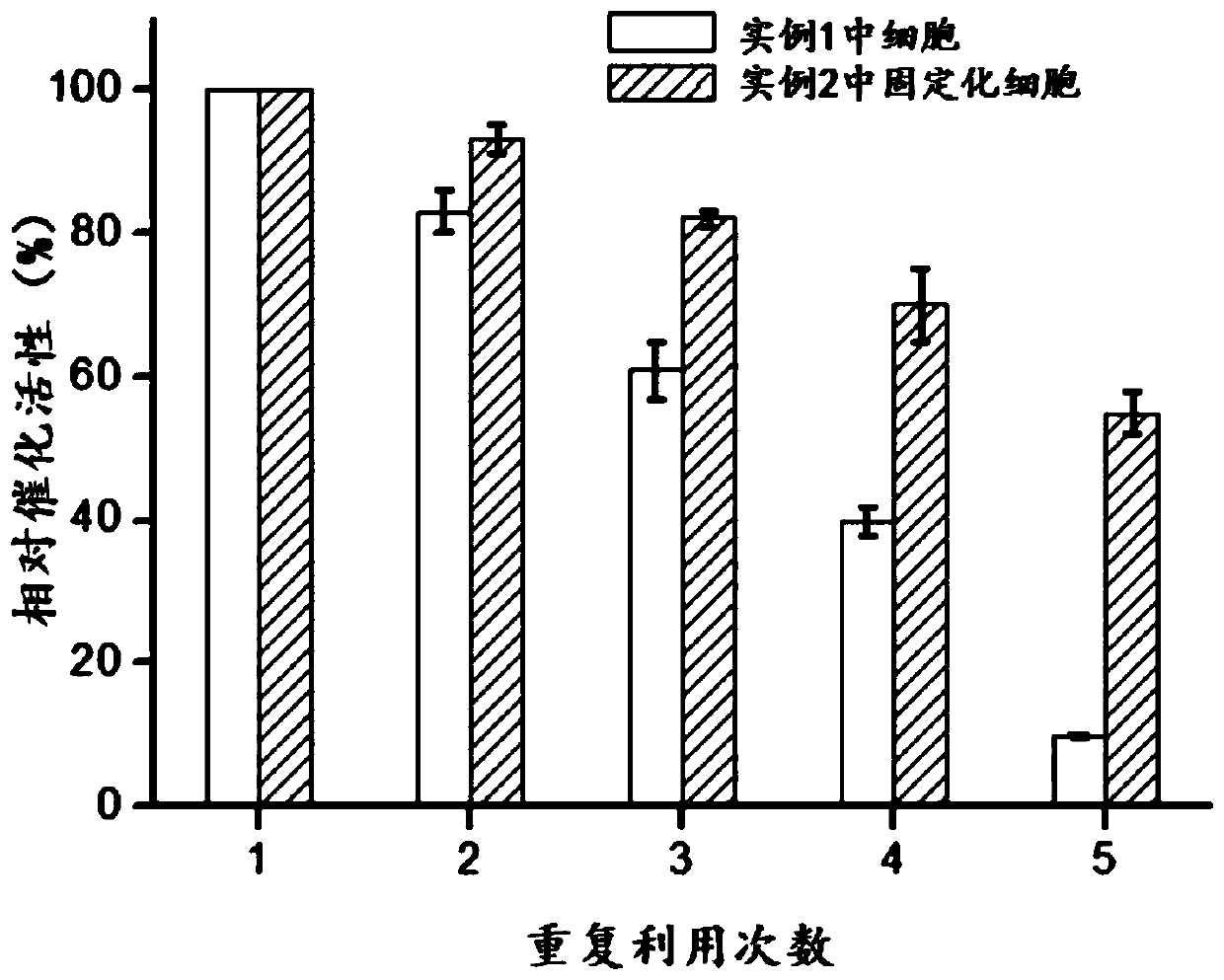
[0070] In 10mL of PBS buffer solution, add the whole cells prepared in Example 1 and the whole cells immobilized by the metal-organic framework material prepared in Example 2, so that the cell concentration is OD 600 =5, 35°C and 200rpm vibration reaction for 10h. After the decarboxylation reaction, the cells were removed by centrifugation, and the supernatant was taken for liquid phase detection.
[0071] 3.2 Method for detecting 3-aminopropionic acid
[0072] Take 100 μL of the sample, add 100 μL of 0.5mol / L sodium bicarbonate solution (pH 9.0), 100 μL of 1% 2,4-dinitrofluorophenylacetonitrile solution in turn, put it in a shaker at 60°C for 1 hour in the dark, and then add 700 μL 0.05mol / L PBS buffer (pH 7.0). 12000rpm high-speed centrifugation for 10min, and the supernatant was taken for liquid phase analysis.
[0073] Liquid phase analysis conditions: the chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com