Dual detection kit for 2019 novel corona virus
A technology of coronavirus and kit, which is applied in the field of primer probe combination and kit for detection of new coronavirus 2019-nCoV, which can solve the problems of CT cross-infection, lack of supporting equipment in epidemic areas, expensive CT equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
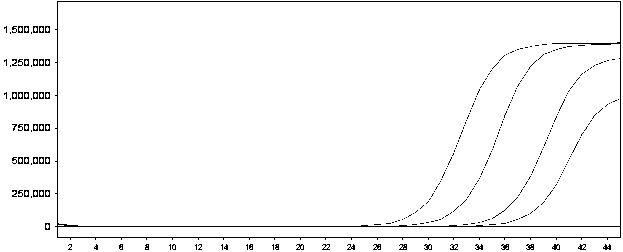
[0115] Example 1 ORF1ab gene and N gene double detection kit detection limit test
[0116] Dilute the ORF1ab gene and N gene pseudoviruses with determined values as initial samples to a concentration of 10 5 copies / ml, serially diluted to 10 4 、10 3 , 500, 250, 100 copies / ml, each concentration sample was added to the final concentration of 10 4 Copies / ml of the plasmid bacteria containing the amplified fragment of the internal standard is used as the sample to be tested, and the sensitivity of the dual detection reagent is tested.
[0117] Test result reference Figure 1-3 :
[0118] figure 1 : ORF1ab gene detection limit;
[0119] figure 2 : N gene detection limit;
[0120] image 3 : internal standard test result.
[0121] The test results showed that the lowest detectable concentration was 250 copies / ml for the detection of positive quality control substances of ORF1ab gene and N gene at different concentrations.
Embodiment 2
[0122] Example 2 ORF1ab gene and N gene double detection kit specificity test
[0123] Use normal saline, SARS virus, rhinovirus, adenovirus, influenza A virus, influenza B virus, parainfluenza virus, coronavirus OC43, respiratory syncytial virus, human coronavirus NL63, human coronavirus HKU1 as specificity Reference product, to test the specificity of ORF1ab gene and N gene double detection kit.
[0124] Test result reference Figure 4-5 :
[0125] Figure 4 : ORF1ab gene and N gene specific test results;
[0126] Figure 5 : ORF1ab gene and N gene-specific detection internal standard test results.
[0127] The test results showed that the test-specific reference products (normal saline, SARS virus, rhinovirus, adenovirus, influenza A virus, influenza B virus, parainfluenza virus, coronavirus OC43, respiratory syncytial virus, human coronavirus NL63 , human coronavirus HKU1), the test results were all negative, and the internal standard quality controls were all positi...
Embodiment 3
[0128] Example 3 ORF1ab gene and N gene double detection kit precision test
[0129] Dilute ORF1ab gene and N gene pseudovirus to 10 5 and 10 3 Copies / ml was used as a precision reference product, each repeated 10 times, and the coefficient of variation of each concentration precision reference product was calculated.
[0130] Test result reference Figure 6-7 :
[0131] Image 6 : ORF1ab gene precision test results
[0132] Figure 7 : N gene precision test result
[0133] The coefficients of variation of the tested ORF1ab gene pseudovirus high concentration and low concentration precision reference products were 0.55% and 0.65% respectively; the variation coefficients of the N gene high concentration and low concentration precision reference products were 0.44% and 0.96% respectively; The coefficients of variation of precision reference products with different concentrations of genetic pseudoviruses were all less than 5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com