Enzyme catalyst and preparation method and application thereof in production of 3-aminopropionic acid
An enzyme catalyst and reaction technology, which is applied in the direction of biochemical equipment and methods, enzymes, lyases, etc., can solve the problems of poor controllability of the catalytic effect of immobilized enzyme catalysts, cumbersome operation process of enzyme catalysts, etc., and achieve good reuse rate, The preparation process is simple and convenient, and the effect of low equipment demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 produces the construction of L-aspartic acid decarboxylase bacterial strain and cultivation process [1~3]
[0041] 1.1 Construction of strains
[0042]Extract the genomes of C.glutamicum ATCC13032, E.coli MG1655, and B.subtilis, and use these three genomes as templates to amplify the ADC gene panD by PCR. The PCR product is recovered from agarose and digested with Nco I and Xho I. , and then use T4 DNA ligase to connect to the pET28a vector that has also undergone double digestion to construct the recombinant plasmid pET28a-panD C.g , Transformed into E.coli BL21(DE3), a single colony was screened out on the Kanna plate.
[0043] 1.2 Medium
[0044] Plate medium (L -1 ): peptone 10g, yeast powder 5g, sodium chloride 5g, agar 20g, kanamycin 0.05g.
[0045] LB medium (L -1 ): peptone 10g, yeast powder 5g, sodium chloride 5g, kanamycin 0.05g.
[0046] Sterilization method: the antibiotics were sterilized by filtration, and the rest were sterilized by hi...
Embodiment 2
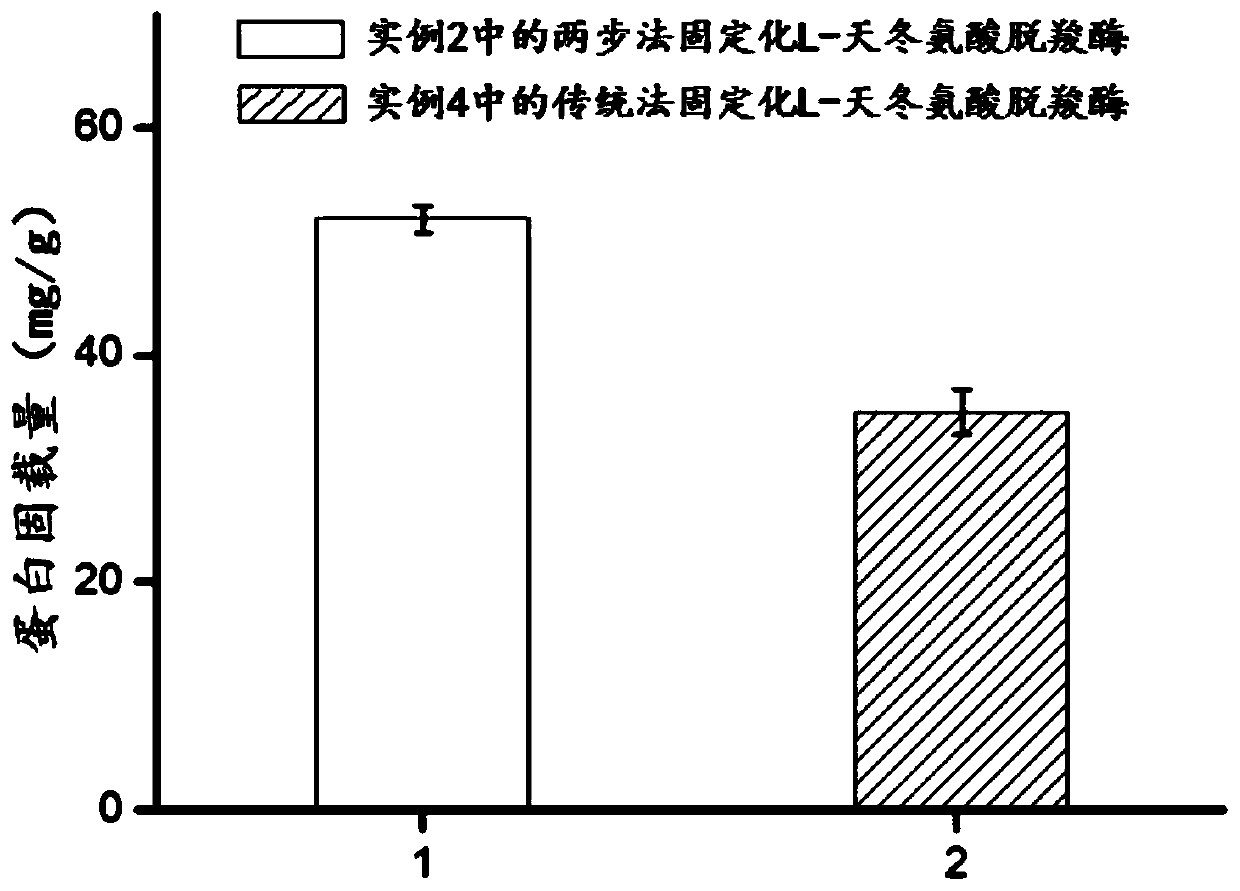
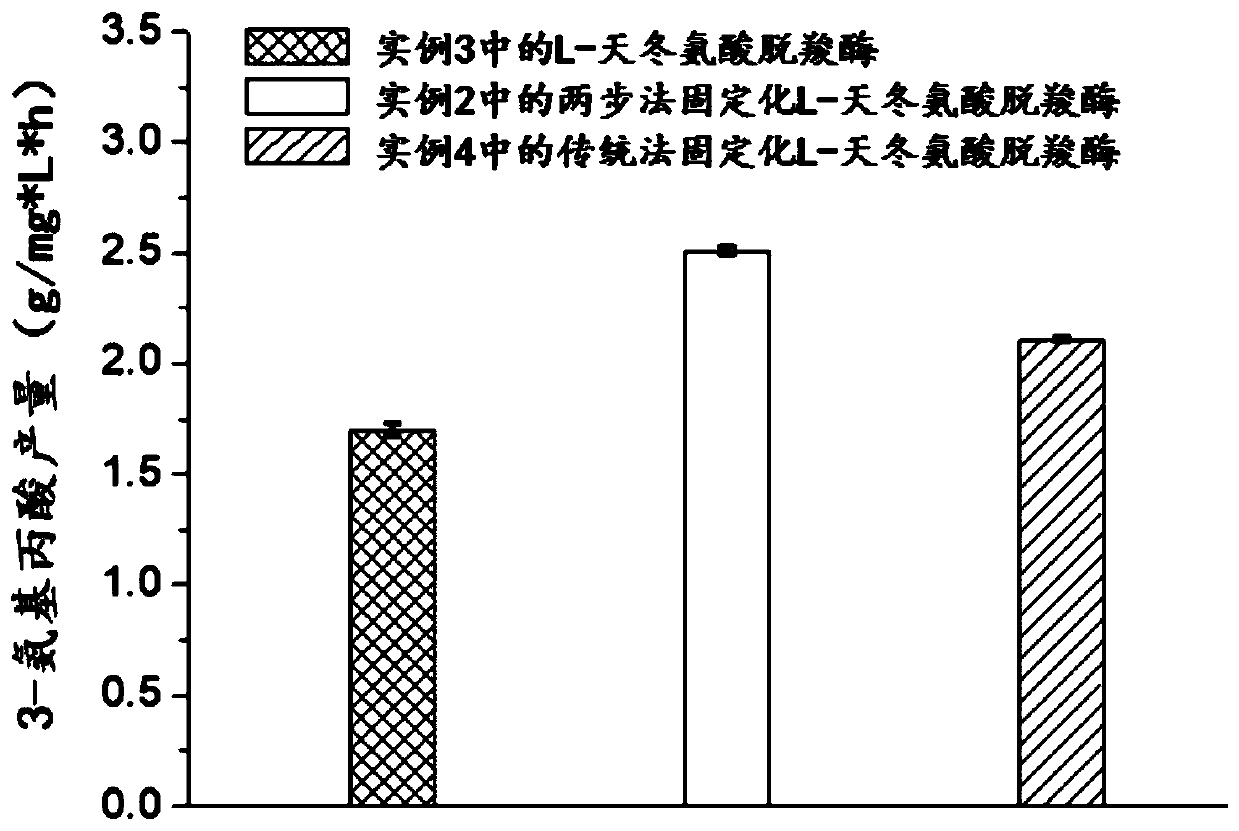
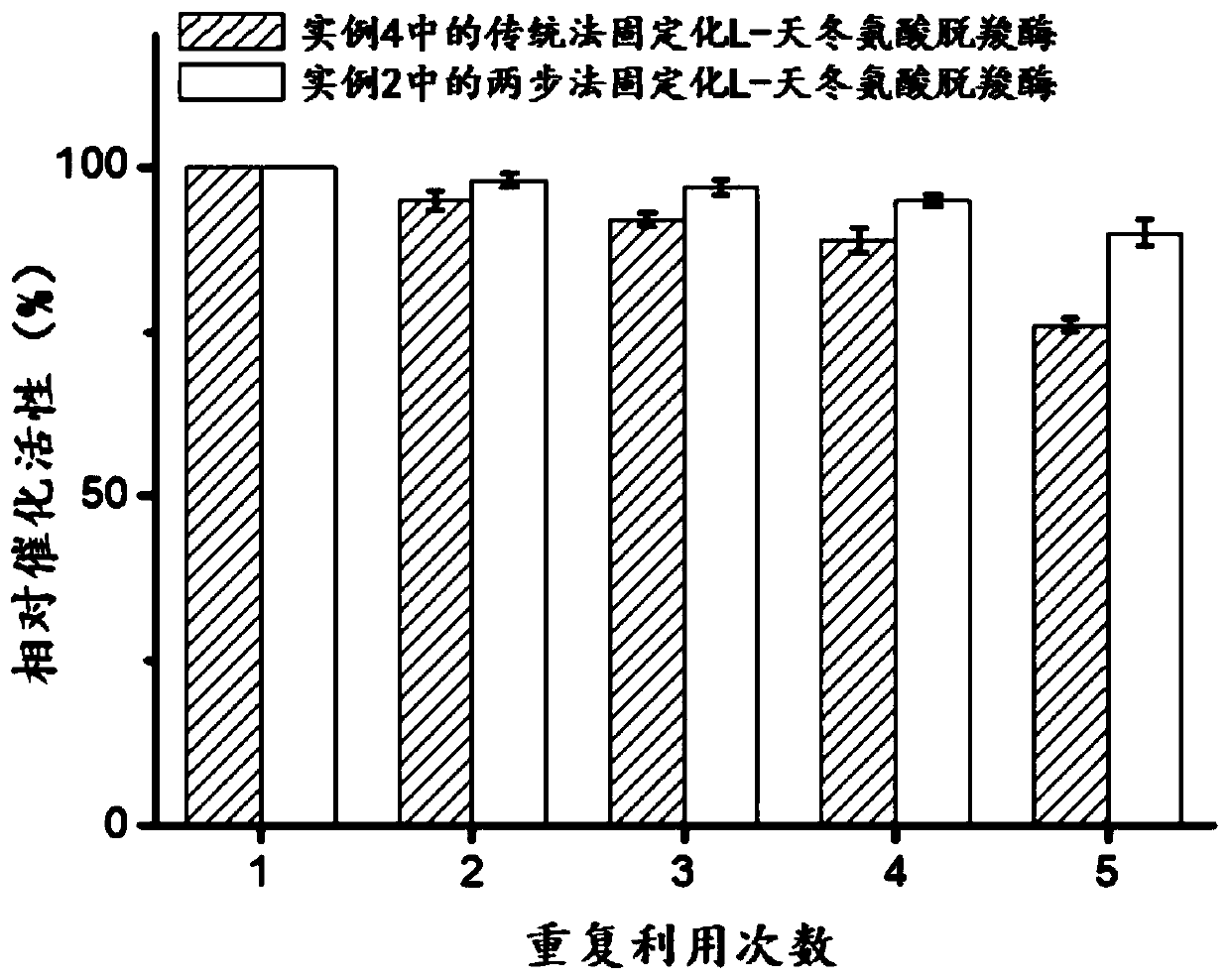
[0056] The preparation process of embodiment 2 two-step method immobilized L-aspartic acid decarboxylase
[0057] 2.1 Preparation of L-aspartate decarboxylase and surfactant mixture
[0058] The thalli that embodiment 1 is collected is formulated into 20mL OD with water 600 =10 resuspension, add 5mL of TX-100 to the resuspension, stir at room temperature at a stirring speed of 700rpm for 45 minutes, then centrifuge at 6000rpm for 10 minutes and take the supernatant to obtain L-aspartic acid Decarboxylase and Surfactant Mixture A.
[0059] 2.2 Preparation of surfactant-modified immobilized L-aspartate decarboxylase
[0060] Measure 20mL of L-aspartate decarboxylase and surfactant mixed solution A, add 10mL 25mmol / L cobalt ion solution and mix for 10 minutes, then add 17mL 100mmol / L dimethylimidazole solution, Stir at 700rpm for 30 minutes, then wash with water and centrifuge to leave the precipitate to obtain surfactant-modified immobilized L-aspartate decarboxylase, which i...
Embodiment 3
[0061] The preparation of embodiment 3L-aspartic acid decarboxylase
[0062] The thalli that embodiment 1 is collected is formulated into 20mL OD with water 600 = 1-10 resuspension liquid, sonicate for 15 minutes, and centrifuge at 3000-6000 rpm to keep the supernatant, which is the L-aspartate decarboxylase solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com