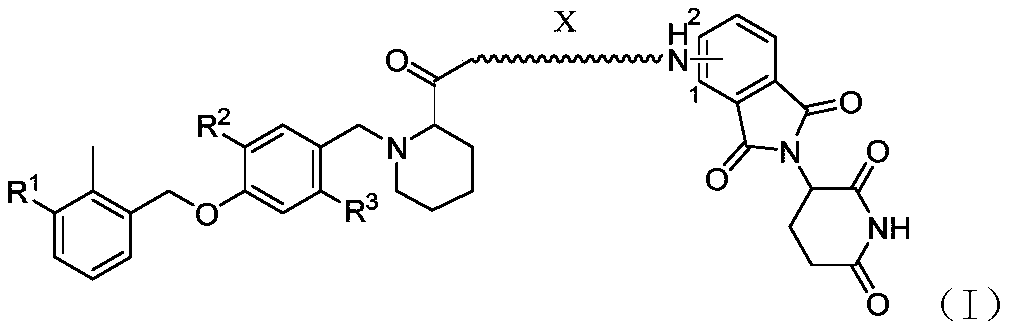
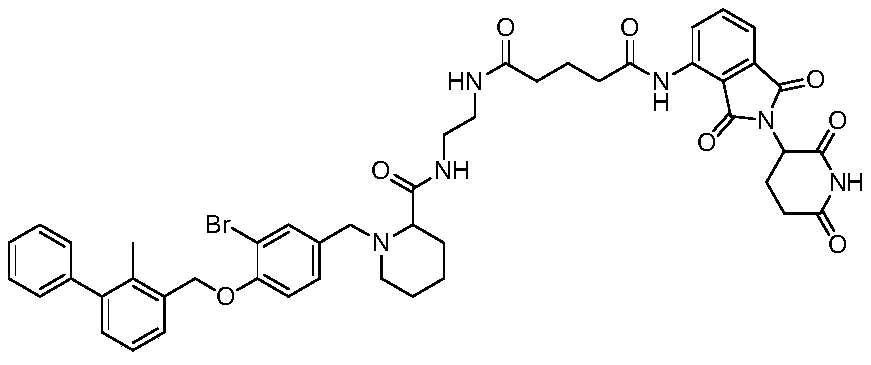
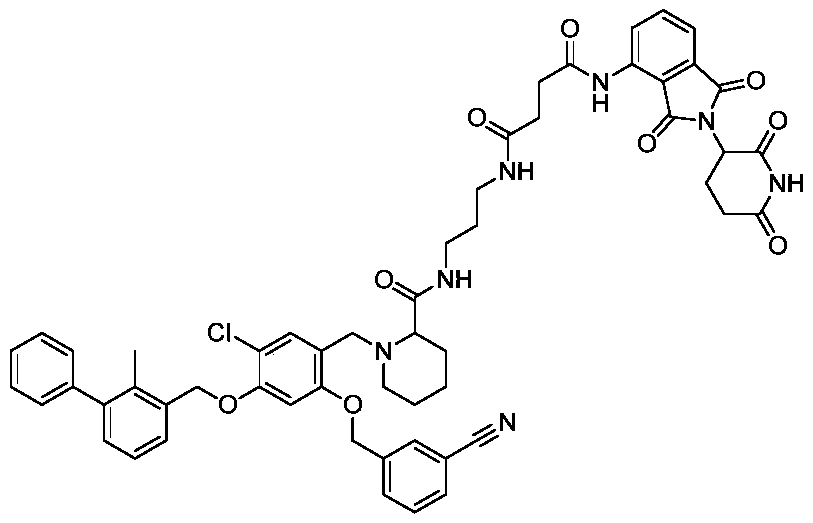
Pomalidomide derivative and application thereof
A technology of pomalidomide and derivatives, applied in the field of pomalidomide derivatives, can solve the problems of unsatisfactory PD-1/PD-L1 inhibition effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 (compound preparation and identification thereof)
[0059] The pomalidomide derivatives of the present invention can be prepared by one of the following methods:
[0060] Preparation method one: aryl benzyl bromide compound (1 equivalent), borate (1.2 equivalent), cesium carbonate (1.2 equivalent) and tetrakistriphenylphosphine palladium (0.1 equivalent) are weighed and placed in dimethyl methylene In a mixed solvent of sulfone and water, the reaction was performed at 100°C for 12 hours after nitrogen replacement three times. After the reaction was completed, the reaction mixture was poured into water, extracted with ethyl acetate, the organic phase was washed with brine three times, dried with anhydrous sodium sulfate, and passed through a silica gel column to obtain the target product.
[0061] Preparation method 2: In an ice bath, add boron tribromide dropwise to the dichloromethane solution of aryl benzyl alcohol. After the reaction was completed, the...
Embodiment 2
[0088] Example 2. Research on the inhibitory effect of a pomalidomide derivative according to the present invention on PD-1 / PD-L1
[0089] The inhibitory effect of the compound of the present invention on PD-1 / PD-L1 is proved by the following method test.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com