Nose comforting tablet and preparation method and use method thereof
A comfortable tablet and comfortable technology, which is applied in the field of rhinitis medicine, can solve the problems of uneven quality of nasal comfort tablets, and achieve the effects of reducing oral drug dosage, improving curative effect, and improving solubility and volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
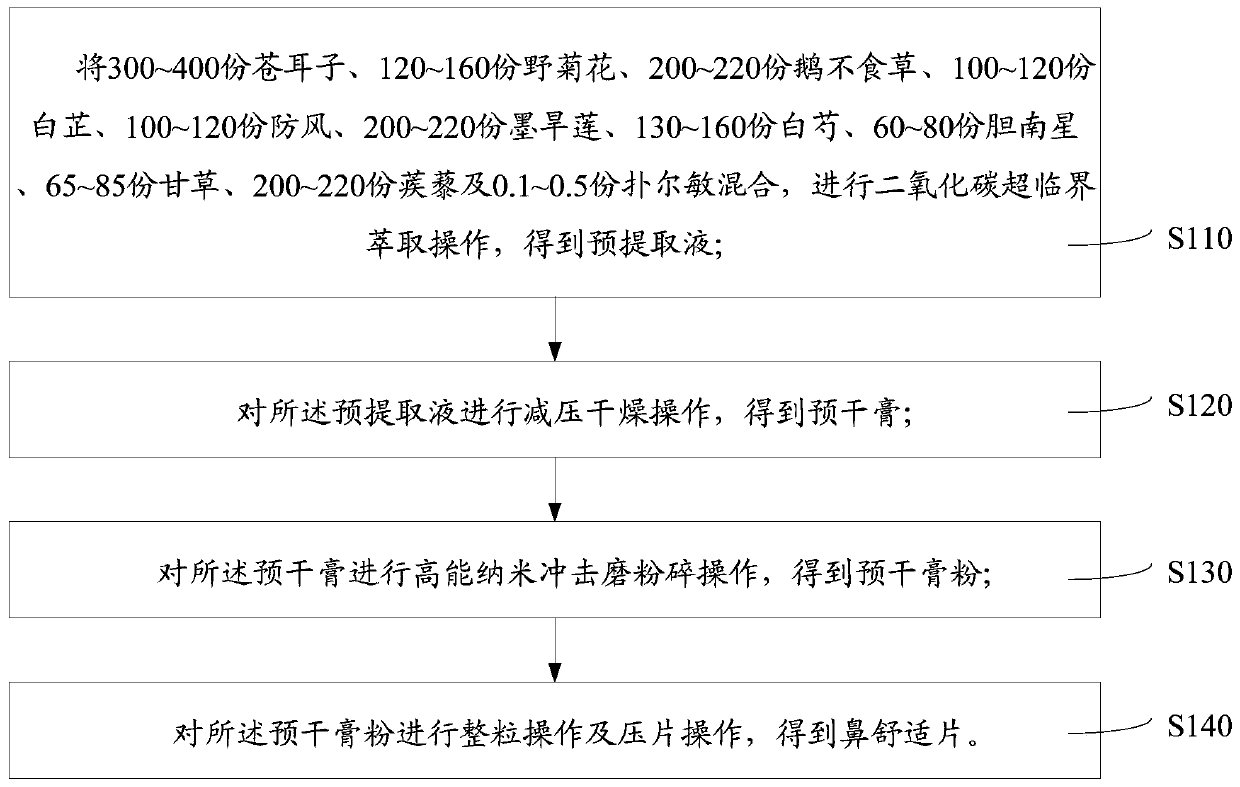
[0033] One embodiment, a nasal comfort tablet, comprising the following traditional Chinese medicine components in parts by mass: 300-400 parts of cocklebur, 120-160 parts of wild chrysanthemum, 200-220 parts of geese not eating grass, 100-120 parts of Angelica dahurica, Fangfeng 100-120 parts, 200-220 parts of Eclipta, 130-160 parts of Paeoniae Alba, 60-80 parts of Dannanxing, 65-85 parts of licorice, 200-220 parts of Tribulus terrestris, and 0.1-0.5 parts of chlorpheniramine. The nasal comfort tablet prepared by the formula has the effects of clearing away heat, reducing inflammation and clearing the mind, and can be used for treating sneezing, runny nose, nasal congestion, headache, allergic rhinitis and chronic sinusitis caused by chronic rhinitis.
[0034] In order to improve the efficacy and quality of the Nasal Comfort Tablet, as another example, the Nasal Comfort Tablet includes the following traditional Chinese medicine components in parts by mass: 364 parts of cockleb...
Embodiment 1
[0055] The preparation method of nasal comfort tablet comprises the following steps: 364g cocklebur, 145g wild chrysanthemum, 218g goose not eating grass, 109g angelica, 109g Fangfeng, 218g black lotus, 145g white peony root, 70g dannanxing, 73g licorice, 218g Tribulus terrestris and 0.3 g of chlorpheniramine were mixed, followed by crushing operation, granulation operation and tabletting operation to obtain nasal comfort tablet.
[0056] The weight of each Nasal Comfort Tablet is 0.2g, orally 3 times a day, 2-3 tablets at a time, and fumigated for external use 2 times a day, 2-3 tablets at a time.
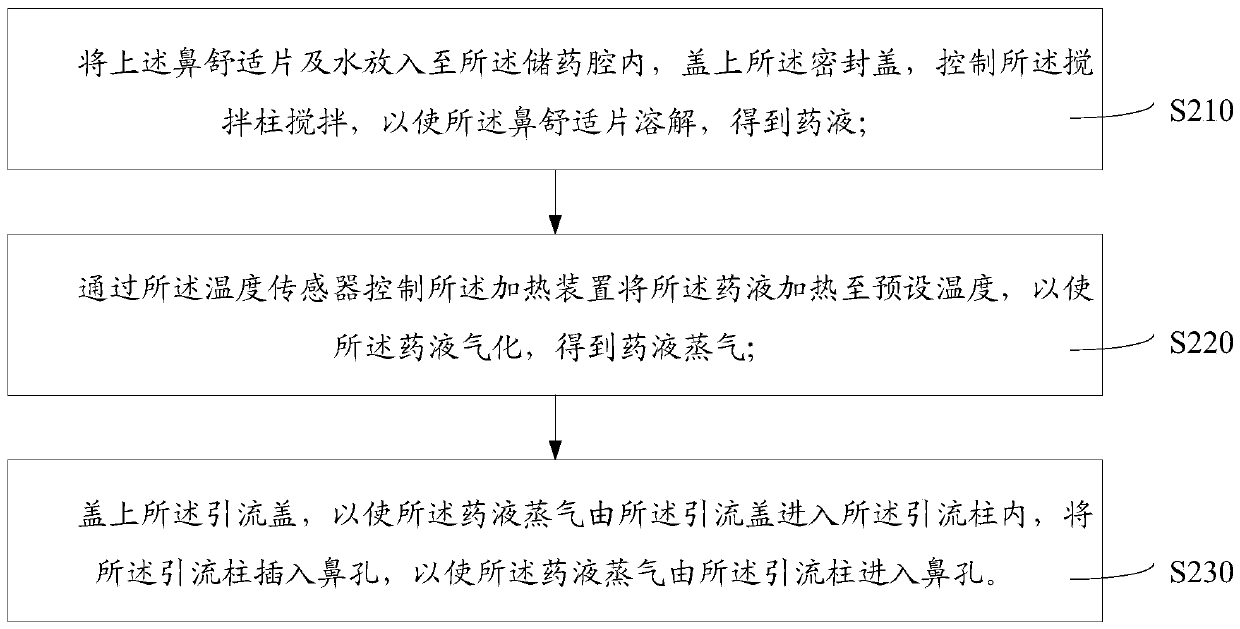
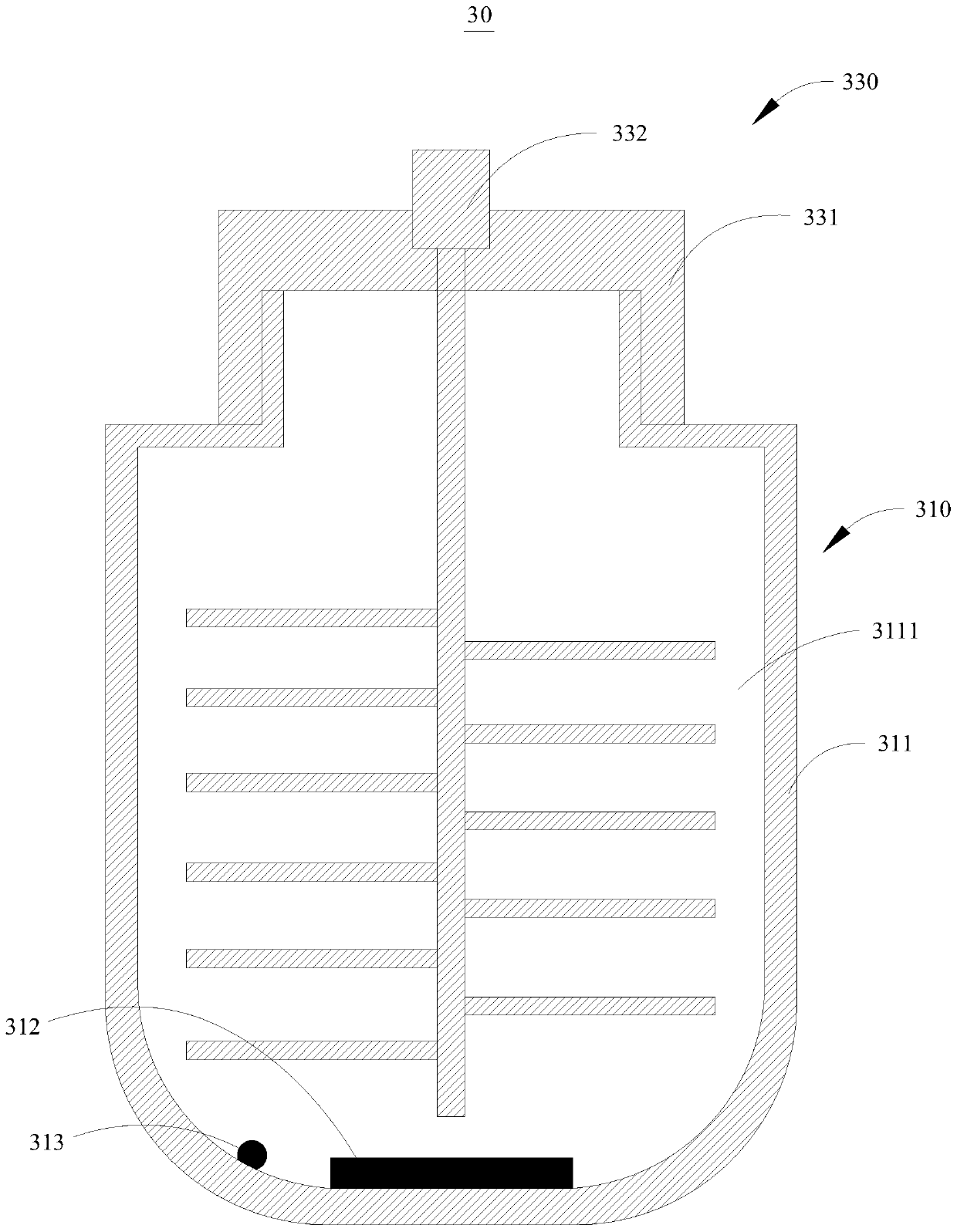
[0057] The method for fumigation of nasal comfort tablets for external use includes the following steps: putting the above-mentioned nasal comfort tablets and water into the medicine storage cavity 3111, covering the sealing cover 331, and controlling the stirring column 332 to stir, so that the The nasal comfort tablet is dissolved to obtain a medicinal liquid; the heating device...
Embodiment 2
[0060] The preparation method of nasal comfort tablet comprises the following steps: 364g cocklebur, 145g wild chrysanthemum, 218g goose not eating grass, 109g angelica, 109g Fangfeng, 218g black lotus, 145g white peony root, 70g dannanxing, 73g licorice, 218g Tribulus terrestris and 0.3g chlorpheniramine are mixed, and carbon dioxide supercritical extraction operation is carried out to obtain a pre-extraction; wherein, the extraction pressure is 30Mpa, the extraction temperature is 30°C, the separator pressure is 20Mpa, and the separator The temperature is 50°C, the separation time is 3h, and the carbon dioxide flow rate is 30L / h; the pre-extraction liquid is subjected to a decompression drying operation to obtain a pre-dry paste; wherein, the decompression drying operation temperature is 70 ℃; carry out high-energy nano-impact milling operation on the pre-dried paste to obtain pre-dried paste powder; wherein, the particle size of the pre-dried paste powder is 250nm; perform g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com