Application of quinine and pharmaceutically acceptable salts thereof to preparation of drug for treating AD (atopic dermatitis)
A technology for atopic dermatitis and drugs, applied in the field of medicine, can solve problems such as benign malaria that cannot be cured, and achieve the effects of expanding medical applications, reducing the area of skin lesions, and reducing pathological damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0048] 1. Experimental method
[0049] 1.1 Experimental grouping
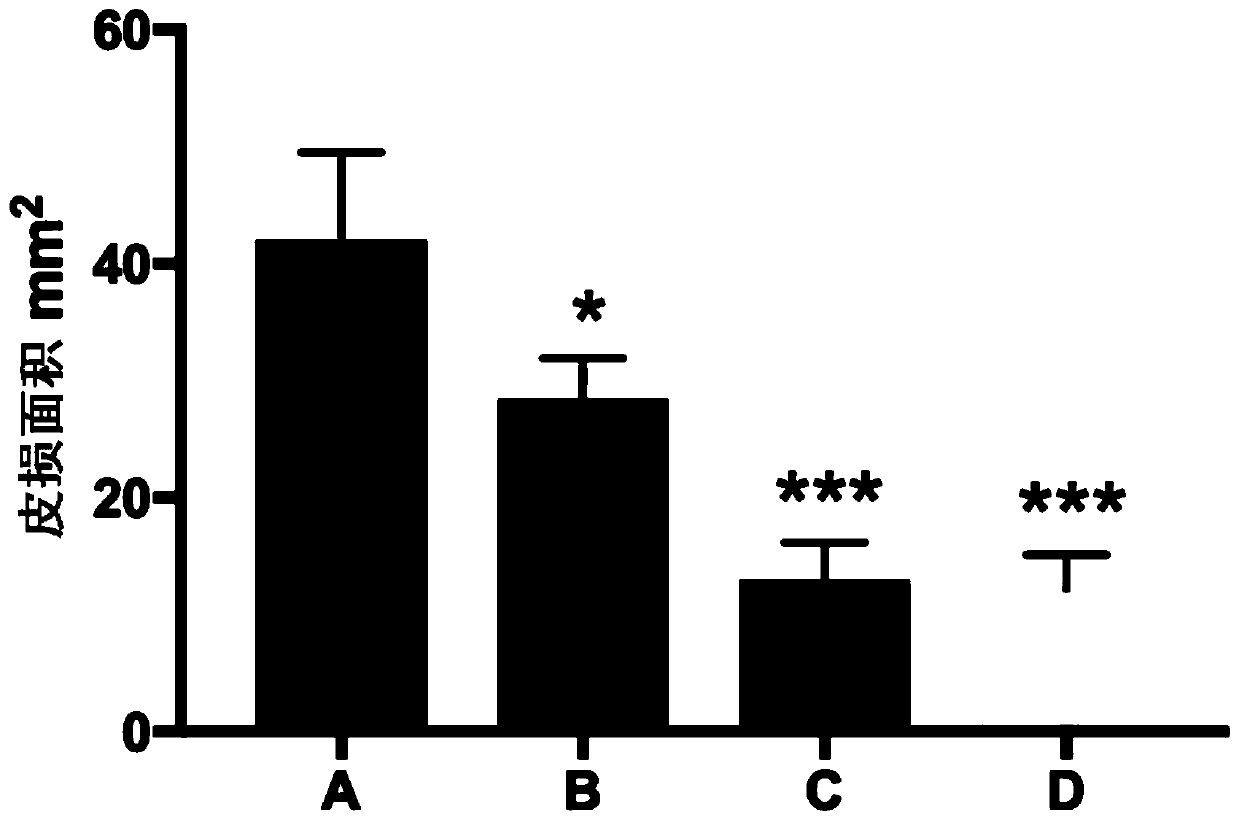
[0050] 24 experimental mice were randomly divided into 4 groups, 6 in each group, which were control group, low-dose group, middle-dose group and high-dose group, which were recorded as group A, group B, group C and group D in turn.
[0051] 1.2 Establishment of AD mouse model
[0052] Shave the back body hair of the mice (use an electric shaver to shave the back long hair first, then apply a layer of depilatory cream, wait for about 3-5 minutes, then soak the gauze with physiological saline and wipe it gently, after the depilation is completed, use a dry Dry the back skin and surrounding hair with gauze), with an area of 2.5cm×2.5cm, apply 0.5% DNFB on the back of the mouse (absorb 100μl 0.5% DNFB with a 100μl pipette gun), and apply it in small amounts (2-3 times) on the back of the mouse On the depilated area (the drug will be dispersed automatically), apply once a day for 28 consecutive days. AD manife...
experiment example 2
[0063] 1. Experimental method
[0064] 1.1 Experimental grouping
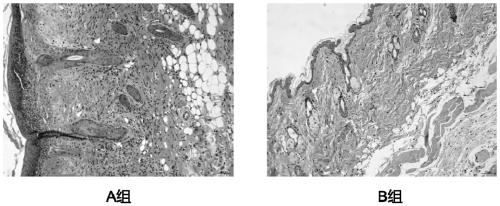
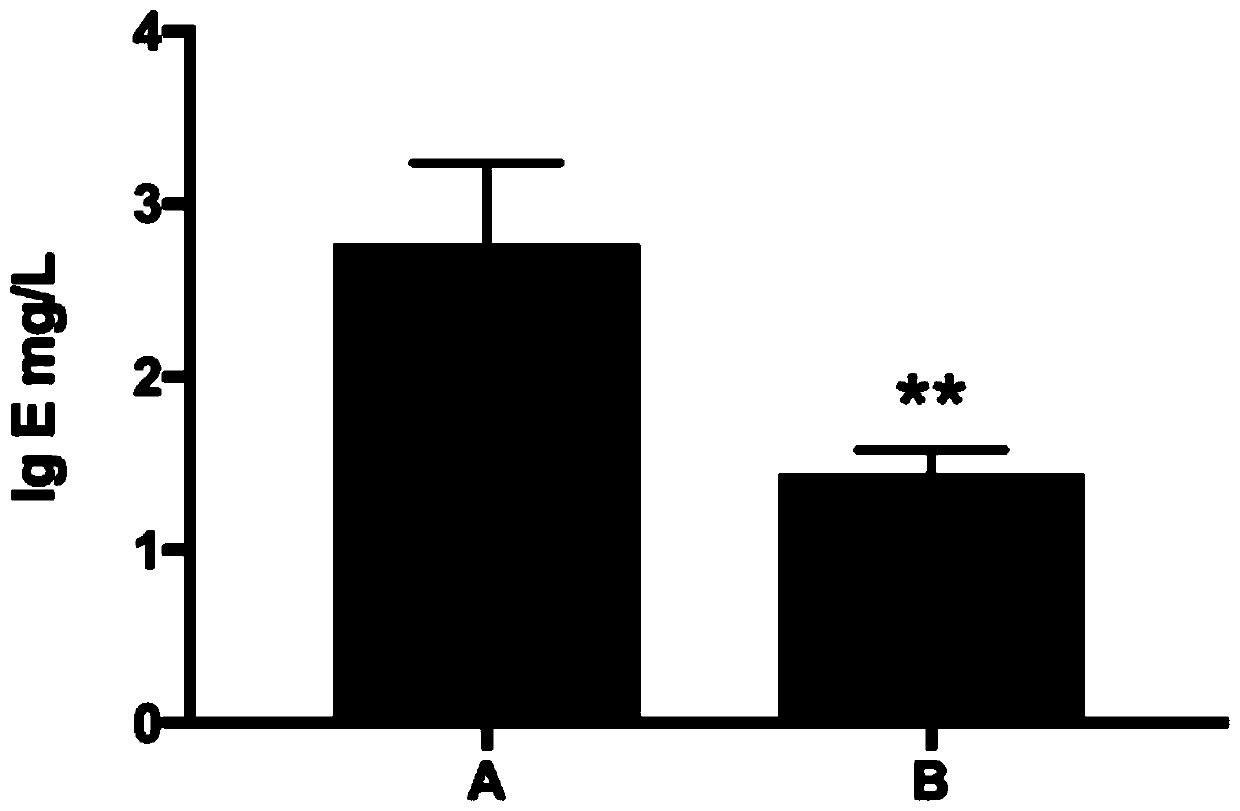
[0065] Twelve experimental mice were randomly divided into two groups, 6 in each group, which were the control group and the experimental group, which were recorded as group A and group B, respectively.
[0066] 1.2 Establishment of AD mouse model
[0067] Same as experimental example 1.
[0068] 1.3 Administration method
[0069] Preparation of the test product: Dissolve quinine in physiological saline so that the concentration of quinine is 10 mg / kg.
[0070] From the 29th day of the experiment, the model mice in each group were treated as follows:
[0071] The model mice in group A were smeared with normal saline on the skin lesion area, once a day, 100 μL each time; the model mice in group B were smeared with the test product on the skin lesion area once a day, 100 μL each time, and each group was treated continuously 7 days.
[0072] 1.4 Histopathological examination of skin lesions
[0073] The spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com