Application of anthocyanin in preparation of medicine for treating cell damage caused by p-aminophenylarsenic acid
A technology of p-aminophenylarsenic acid and cell damage, which can be used in drug combinations, pharmaceutical formulations, plant raw materials, etc., can solve the problems of anthocyanin cell apoptosis and other problems that have not yet been seen, and achieve the effect of expanding medical uses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of Solanum solanum anthocyanins
[0029] (1) Remove the petiole of the fresh nightshade fruit and wash it, beat it with a juicer to obtain a slurry.
[0030] (2) Use hydrochloric acid aqueous solution with pH=1 as the extraction solvent, and ultrasonically extract the slurry, the quality of the extraction solvent and the slurry is 1:6, the ultrasonic power is 600W, ultrasonic 3 times, each time 30min, after three times, use 200 mesh gauze Filter to obtain the crude extract of solanum anthocyanins.
[0031] (3) Mix the crude extract of Solanum solanum anthocyanin with ethyl acetate at a volume ratio of 1:3, perform the first extraction after fully shaking and mixing, and collect the lower aqueous solution; then mix the lower aqueous solution with ethyl acetate in a volume ratio 1:3 for mixing, fully oscillating and mixing for the second extraction, collecting the aqueous solution of the lower layer to obtain the extract of Solanum solanum a...
Embodiment 2
[0034]Embodiment 2: CCK-8 method detects cell viability
[0035] 1. Test method:
[0036] The cell viability was detected by CCK-8 method to study the effect of p-aminophenylarsenic acid and anthocyanin on the apoptosis of DF-1 cells. The anthocyanins are the solanum anthocyanins prepared in Example 1.
[0037] Culture DF-1, and when the cell fusion degree reaches more than 80%, the cells (5×10 5 cells / well) to a 96-well plate (100 μL per well) and cultured for 24 h. The experiment was divided into three treatments, namely: control group, ASA group, NA and ASA co-incubation group; among them, the control group was a blank control without treatment; the ASA group was treated with 0-100mM / L p-aminobenzene arsenic acid Cells for 24 hours; NA and ASA co-incubation group treated cells with 10mM / L p-aminophenyl arsenic acid and 0-200μg / ml anthocyanin for 24 hours.
[0038] After 24 hours, 10 μL CCK-8 reagent was added to each well. Incubate in the incubator for 2h. Be careful ...
Embodiment 3
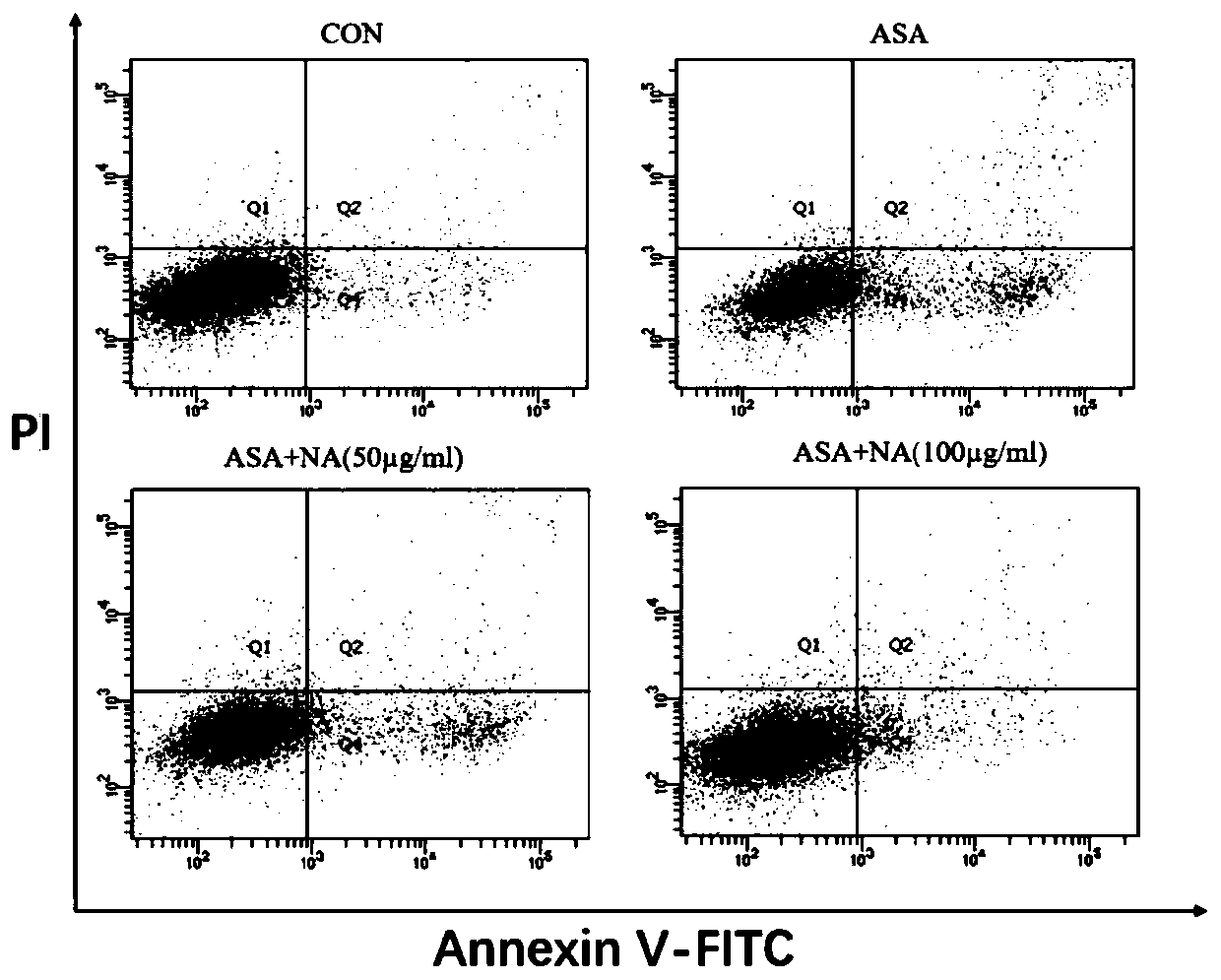
[0044] Example 3: Detection of cell apoptosis rate by flow cytometry
[0045] 1. Test method:
[0046] (1) Cell culture: DF-1 cells were cultured until the confluence of the cells reached more than 80%, and then subcultured to a 6-well plate and cultured for 24 hours.
[0047] (2) Cell poisoning: the experiment was divided into four groups, control group, p-aminophenylarsenic acid (ASA) challenge group, ASA (10mM) alone group, anthocyanin (NA) (50μg / mL)+ASA (10mM ) co-incubation group, NA (100μg / mL)+ASA (10mM) co-incubation group, the exposure time was 24h. The anthocyanins are the solanum anthocyanins prepared in Example 1.
[0048] (3) Cell collection: absorb the old culture medium and store it in a centrifuge tube, wash the cells twice with PBS, collect the cleaning solution in a centrifuge tube, digest the cells with EDTA-free trypsin, and control the digestion time at 5-7 minutes. Cells were collected upon completion of digestion. Centrifuge at 1000rpm for 3min, disca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com