Fused ring pyrazolone derivative and preparation method thereof
A kind of technology of cyclopyrazolone and cyclopyrazolone, which is applied in the field of cyclopyrazolone derivative and preparation thereof, and can solve the limitation of synthesis and development of cyclopyrazolone, large demand of catalyst and raw material. Restricted sources and other issues, to achieve the effects of excellent pharmacological activity, high stability, and simple and easy-to-obtain reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The invention provides a kind of preparation method of cyclic pyrazolone derivative, and described preparation method comprises:
[0033] (1) [Cp*IrCl 2 ] 2 , AgOAc, pyrazolone compound, azide compound and solvent mixed reaction;
[0034] (2) The cyclic pyrazolone derivatives were obtained by following the reaction by TLC and separating by column chromatography.
[0035] In a preferred embodiment of the present invention, the pyrazolone compound is selected from N-phenyl-2-trifluoromethyl-pyrazolone, N-(3-methylphenyl)-2- Trifluoromethyl-pyrazolone, N-(3,5-dimethylphenyl)-2-trifluoromethyl-pyrazolone, N-(4-methylphenyl)-2-tri Fluoromethyl-pyrazolone, N-(4-ethylphenyl)-2-trifluoromethyl-pyrazolone, N-(4-methoxyphenyl)-2-trifluoromethyl -pyrazolone, N-(4-isopropylphenyl)-2-trifluoromethyl-pyrazolone or N-(4-tert-butylphenyl)-2-trifluoromethyl-pyridine One of the oxazolinones.
[0036] In a preferred embodiment of the present invention, the azide compound is selected...
Embodiment 1
[0048] Add [Cp*IrCl 2 ] 2 (0.01 mmol), AgOAc (0.05 mmol), N-phenyl-2-trifluoromethyl-pyrazolone (0.25 mmol), benzoyl azide (0.5 mmol), 1,2-dichloroethane (3mL), reacted at 70°C for 8h, followed the reaction by TLC, after the reaction, extracted, dried, and separated by column chromatography (developing agent petroleum ether / ethyl acetate v / v=8:1), that is, the yield of 76% Obtained pure white solid compound A1;
[0049]
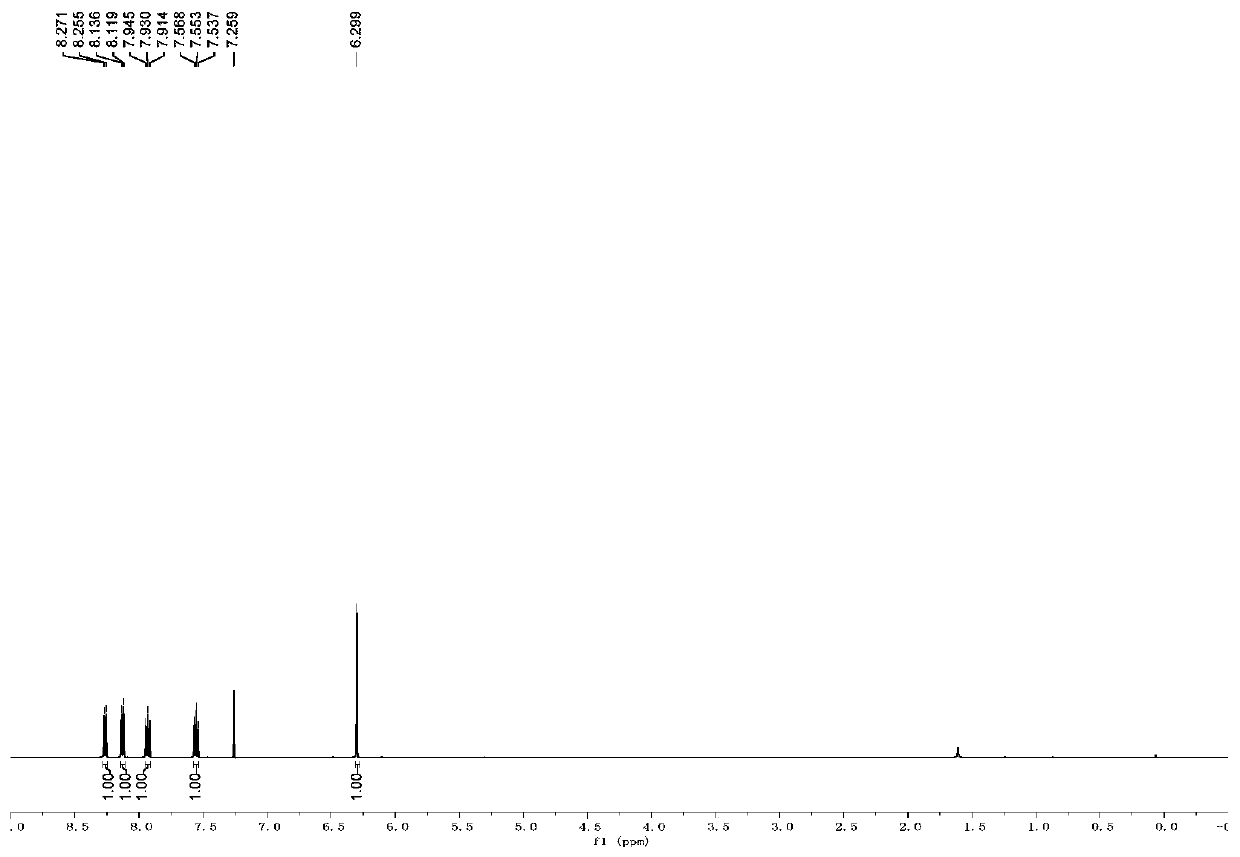
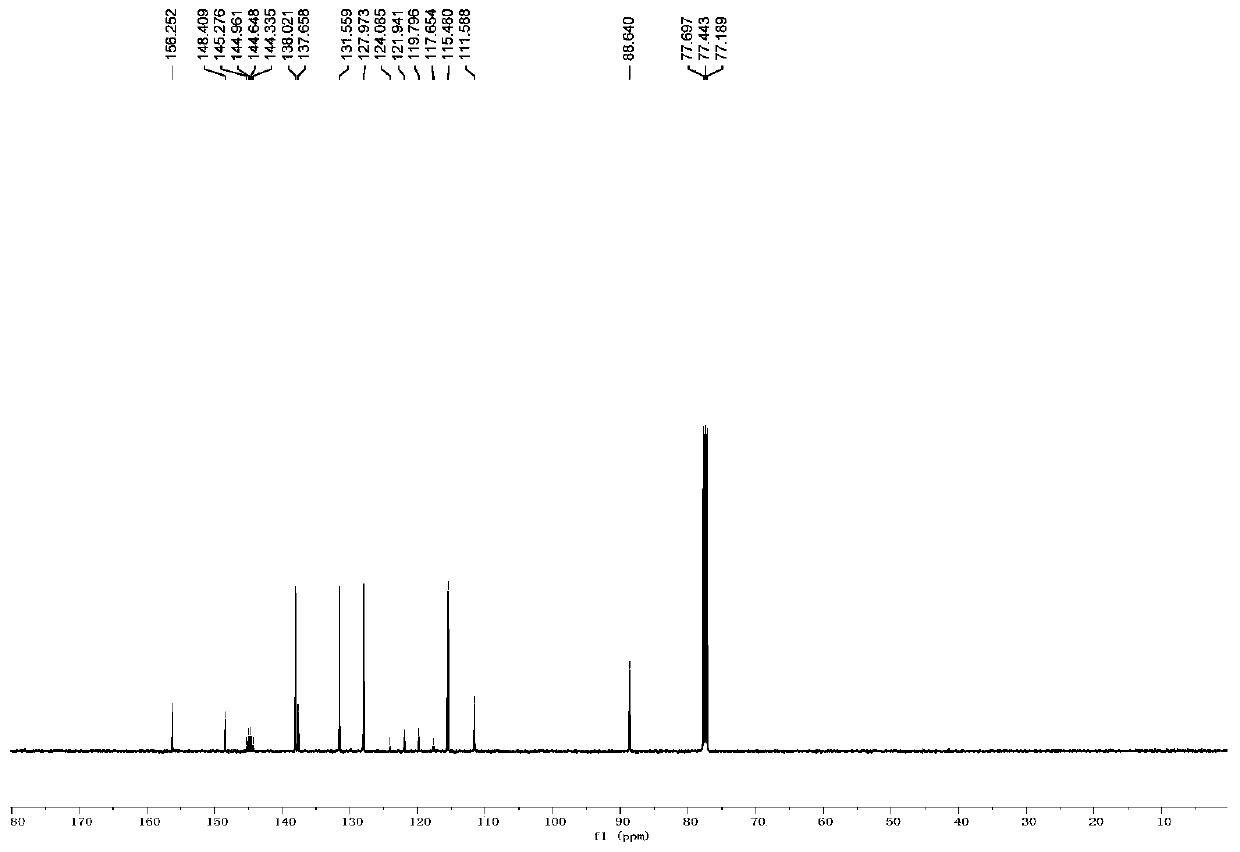
[0050] The H NMR and C NMR data of compound A1 are as follows:
[0051] 1 H NMR (500MHz, CDCl 3 )δ8.26(d, J=8.0Hz, 1H), 8.12(d, J=8.5Hz, 1H), 7.93(t, J=7.5Hz, 1H), 6.29(s, 1H);
[0052] 13 C NMR (125MHz, CDCl 3 )δ156.2, 148.4, 144.8 (q, J C-F =39.1Hz), 138.0, 137.6, 131.5, 127.9, 120.8 (q, J C-F =268.0Hz), 115.4, 111.5, 88.6;
[0053] It can be seen that the single peak at 6.29 ppm and the double peak at 8.26 ppm in the proton NMR spectrum can confirm the formation of the target product.
Embodiment 2
[0055] Add [Cp*IrCl 2 ] 2 (0.01mmol), AgOAc (0.05mmol), N-(3-methylphenyl)-2-trifluoromethyl-pyrazolone (0.25mmol), benzoyl azide (0.5mmol), 1, 2-Dichloroethane (3mL), reacted at 70°C for 8h, followed the reaction by TLC, after the reaction, extracted, dried, and separated by column chromatography (developer petroleum ether / ethyl acetate v / v=8:1), that is A pure white solid compound A2 can be obtained in a yield of 71%;
[0056]
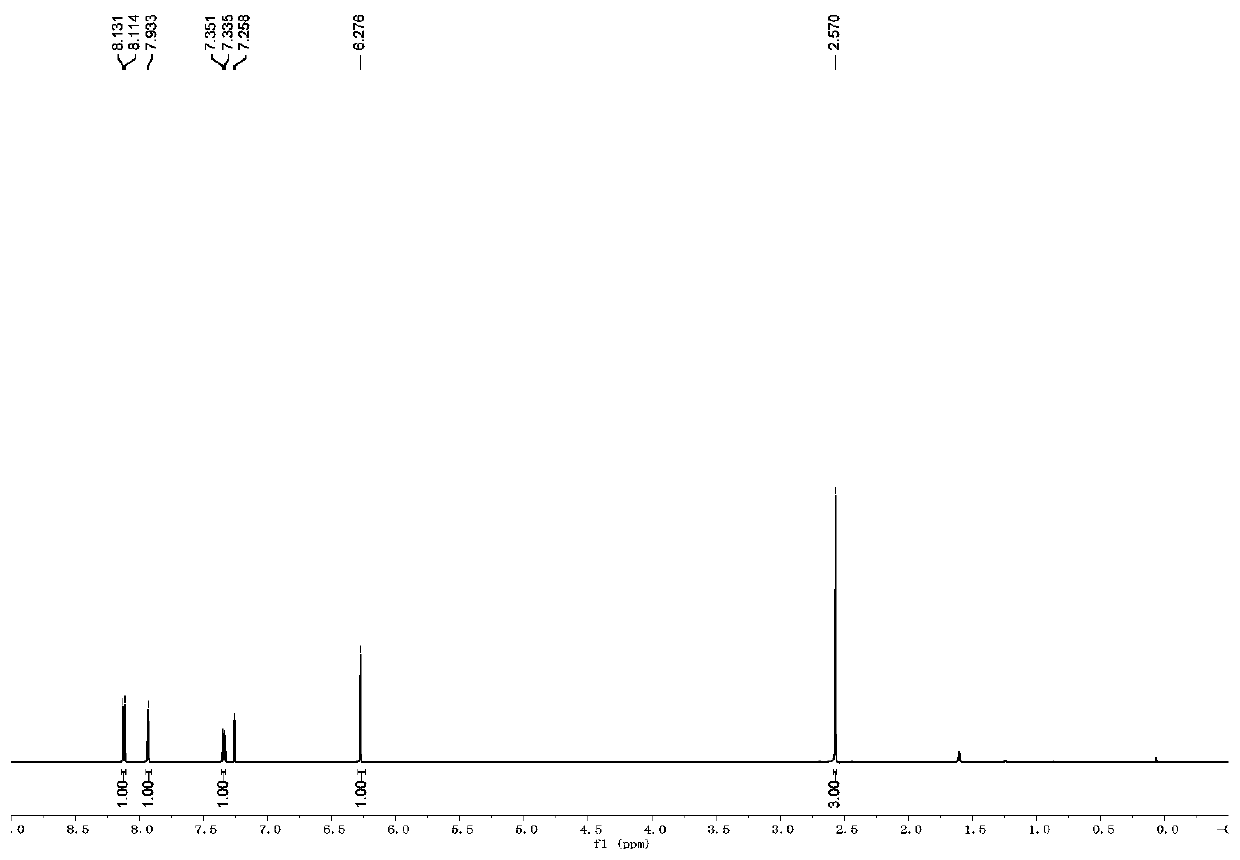
[0057] The H NMR and C NMR data of compound A2 are as follows:
[0058] 1 H NMR (500MHz, CDCl 3 )δ8.12(d, J=8.5Hz, 1H), 7.93(s, 1H), 7.34(d, J=8.0Hz, 1H), 6.27(s, 1H), 2.57(s, 3H);
[0059] 13 C NMR (125MHz, CDCl 3 )δ156.3, 150.3, 148.5, 144.6 (q, J C-F =39.1Hz), 137.5, 131.4, 129.1, 120.9 (q, J C-F =267.8Hz), 115.4, 108.9, 88.5, 22.8;
[0060] It can be seen that the single peak at 6.27ppm and the double peak at 8.12ppm in the proton NMR spectrum can confirm the formation of the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com