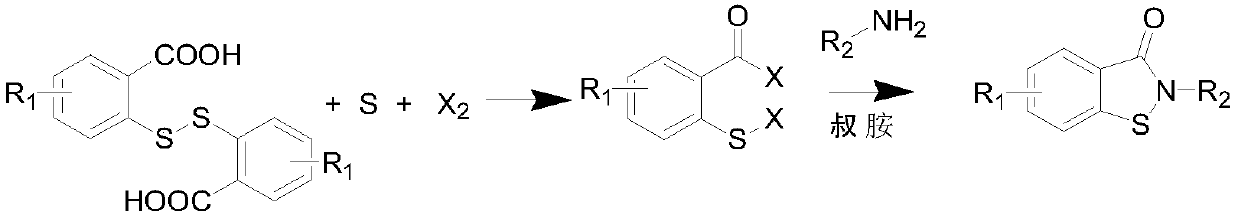
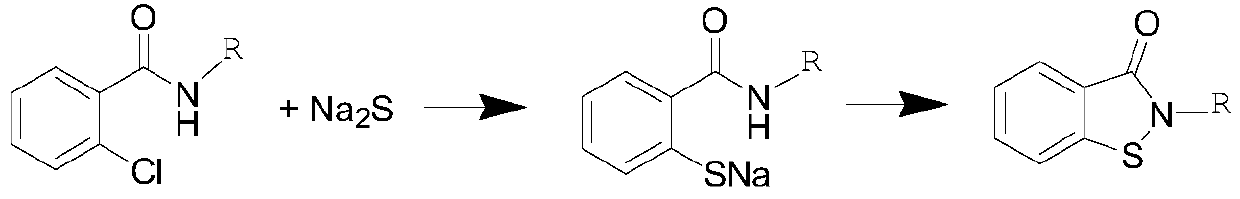
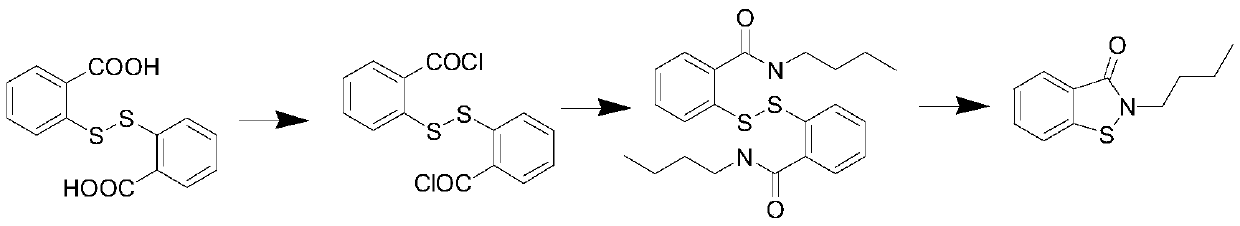
Novel synthesis method of N-substituted benzisothiazoline-3-ketone derivative
A technology of benzisothiazoline and ketone derivatives, which is applied in the direction of organic chemistry, can solve the problems of unfavorable environmental protection, high reaction temperature, and many by-products, and achieve improved atom economy, high economic benefits, and reduced emissions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 30.6 grams of dithiosalicylic acid, 3.5 grams of sulfur, 200 ml of toluene was added, the temperature was raised to 40 ° C, and 28.4 grams of chlorine gas was introduced until the reaction liquid became clear. TLC detected that the conversion of the raw materials was complete, and the solvent was removed under reduced pressure to obtain a yellow oil The object was 44.5 grams, and solidified into a yellow solid after cooling down. Yield 99%, content 97%; this is the intermediate CTBC.
Embodiment 2
[0036] 30.6 grams of dithiosalicylic acid, 2.3 grams of sulfur, 200 ml of chlorobenzene was added, the temperature was raised to 60 ° C, 18.5 grams of chlorine gas was introduced, TLC detected that the raw materials disappeared, and the solvent was removed under reduced pressure to obtain 37.6 grams of a yellow solid, with a yield of 90%. , with a content of 95%, is an intermediate CTBC.
Embodiment 3
[0038]30.6 grams of dithiosalicylic acid, 2.9 grams of sulfur, 200 ml of ethylene dichloride was added, the temperature was raised to 50 ° C, 21.5 grams of chlorine gas was introduced, the reaction solution became clear, and the solvent was removed under reduced pressure to obtain 40.2 grams of a yellow solid. The yield 96%, content 97%, is CTBC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com