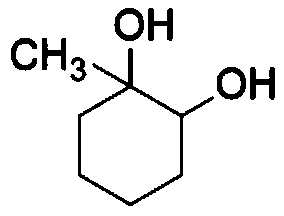
Synthesis method of cyclohexanol derivative 1-methyl-1, 2-cyclohexanediol
A synthesis method and technology of cyclohexanediol, which is applied in the field of catalytic conversion and utilization of biomass resources, can solve the problems of high catalyst requirements and harsh reaction conditions, and achieve the effects of excellent reaction effect, easy reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Prepare carbon carrier from biomass tar: Weigh 20g of carbon precursor biomass tar and dissolve it in 100ml absolute ethanol, and then add 100ml 0.2mol / L activator KOH aqueous solution dropwise to the biomass tar ethanol solution. Stir in a water bath at °C for 8 hours until a uniform solid is formed, and then dry at 100 °C for 12 hours to obtain a solid mixture. Grind the solid mixture into uniform fine powder, put it in a tube furnace, heat up to 800°C at 1°C / min in a nitrogen atmosphere, and calcine for 2h. After cooling to room temperature, use HCl (1M, 100ML) to condense and reflux the sample in a water bath environment for 10 hours, then rinse with deionized water twice and ethanol for three times. Finally, vacuum drying was carried out at 100°C for 12 hours to prepare a low-cost carbon support.
[0044] The preparation of the catalyst, load the metal element nickel with a mass fraction of 10%: 1.1010g Ni(NO 3 ) 2 ·6H 2 O is dissolved in 250ml of deionized water...
Embodiment 2
[0046] (1) Prepare carbon carrier with biomass tar: weigh 20g of carbon precursor biotar and dissolve in 100ml of absolute ethanol, and then add 100ml of 0.1mol / L activator K 2 FeO 4 The aqueous solution was added dropwise to the biological tar solution, stirred in a water bath at 60°C for 8 hours until a uniform solid, and then dried at 100°C for 12 hours to obtain a solid mixture. Grind the solid mixture into a uniform fine powder, put it in a tube furnace, and calcine at 800°C for 2h in a nitrogen atmosphere. After cooling to room temperature, use HCl (1mol / L, 100mL) to condense and reflux the sample in a water bath environment for 10 hours, then rinse with deionized water twice and ethanol for three times. Finally, vacuum drying was carried out at 100°C for 12 hours to prepare a low-cost carbon support.
[0047] The preparation of the catalyst: load the metal element nickel with a mass fraction of 10%, add 1.1010g Ni(NO 3 ) 2 ·6H 2 O is dissolved in 250ml of deionized water, ...
Embodiment 3
[0049] Add 0.2g of guaiacol, 20ml of dodecane, and 0.1g of the 10wt% nickel catalyst obtained in Example 1 into the reactor at the same time, add 2MPa hydrogen gas, stir with the magnet, 600r / min, adjust The reaction temperature was 180°C, 190°C, 220°C, 260°C, and the reaction time was 2 hours. The results are shown in Table 1.
[0050] Table 1 Yield of 1-methyl-1,2-cyclohexanediol
[0051] Reaction temperature (℃) Yield of 1-methyl-1,2-cyclohexanediol (%) 22077.6
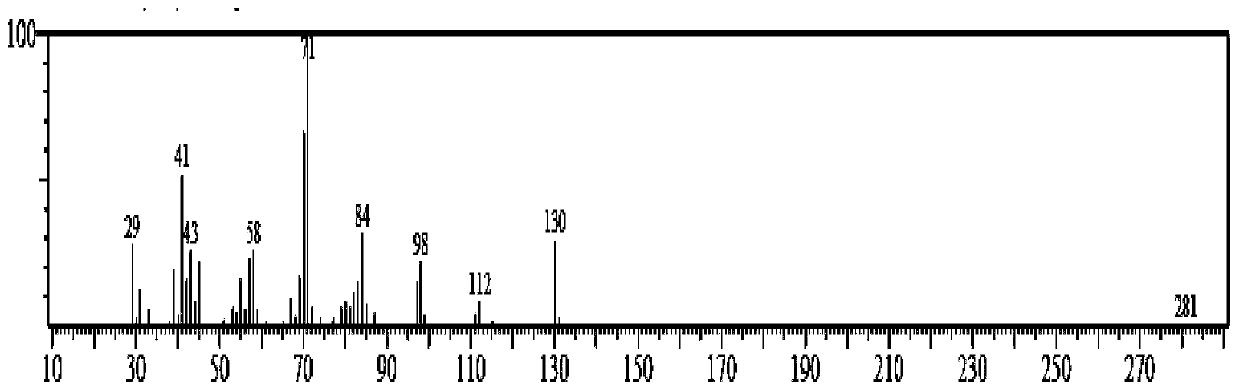
[0052] From figure 1 Analyzed by the gas chromatography-mass spectrogram, the detected substance has the highest similarity with 1-methyl-1,2-cyclohexanediol, and passed figure 2 Examination of the gas chromatogram showed the presence of double peaks on both spectra, which confirmed that the reaction produced 1-methyl-1,2-cyclohexanediol. The double peaks were due to 1-methyl The presence of cis and trans isomers of 1,2-cyclohexanediol was detected by gas chromatography at the same time, and the internal standard m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com