Covalent organic framework material with benzofuran structure, synthesis method and application thereof
A technology of covalent organic framework and benzofuran, which is applied in the field of synthesis and covalent organic framework materials, can solve the problems of restricting the development of new structures and new materials, limited reaction types, etc., and achieves a simple synthesis method, good operability, and huge size. The effect of applying value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
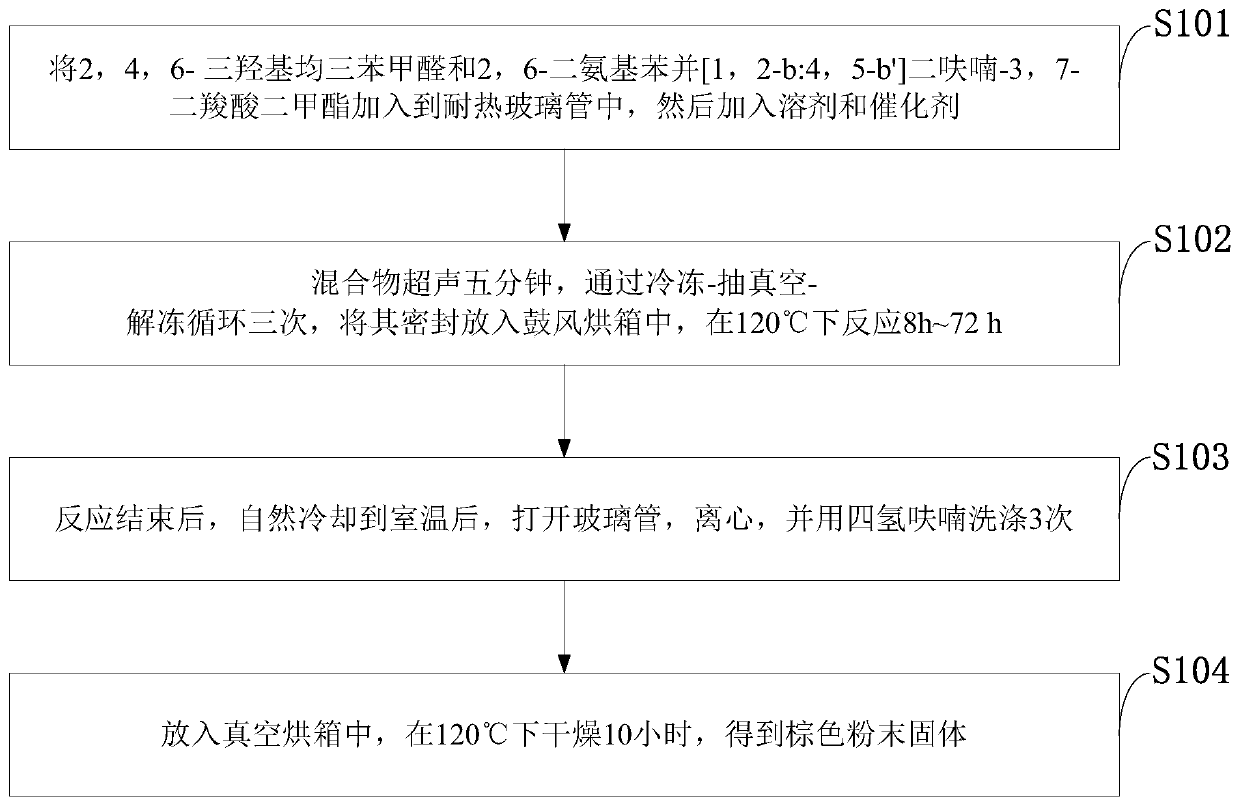
[0039] Such as figure 1 As shown, the synthesis method of the covalent organic framework material with benzofuran structure provided by the embodiment of the present invention includes the following steps:
[0040] S101: Mix 2,4,6-trihydroxy-triphenylcarbaldehyde and 2,6-diaminobenzo[1,2-b:4,5-b']difuran-3,7-dicarboxylic acid dimethyl The ester is added to the heat-resistant glass tube, and then the solvent and catalyst are added;
[0041] S102: Sonicate the mixture for five minutes, cycle through freezing-vacuumizing-thawing three times, seal it and put it in a blast oven, and react at 120°C for 8h-72h;
[0042] S103: After the reaction is finished, cool down to room temperature naturally, open the glass tube, centrifuge, and wash with THF 3 times;
[0043] S104: put in a vacuum oven, and dry at 120° C. for 10 hours to obtain a brown powder solid.
[0044] The synthesis method of the covalent organic framework material with the benzofuran structure provided by the embodime...
Embodiment 1
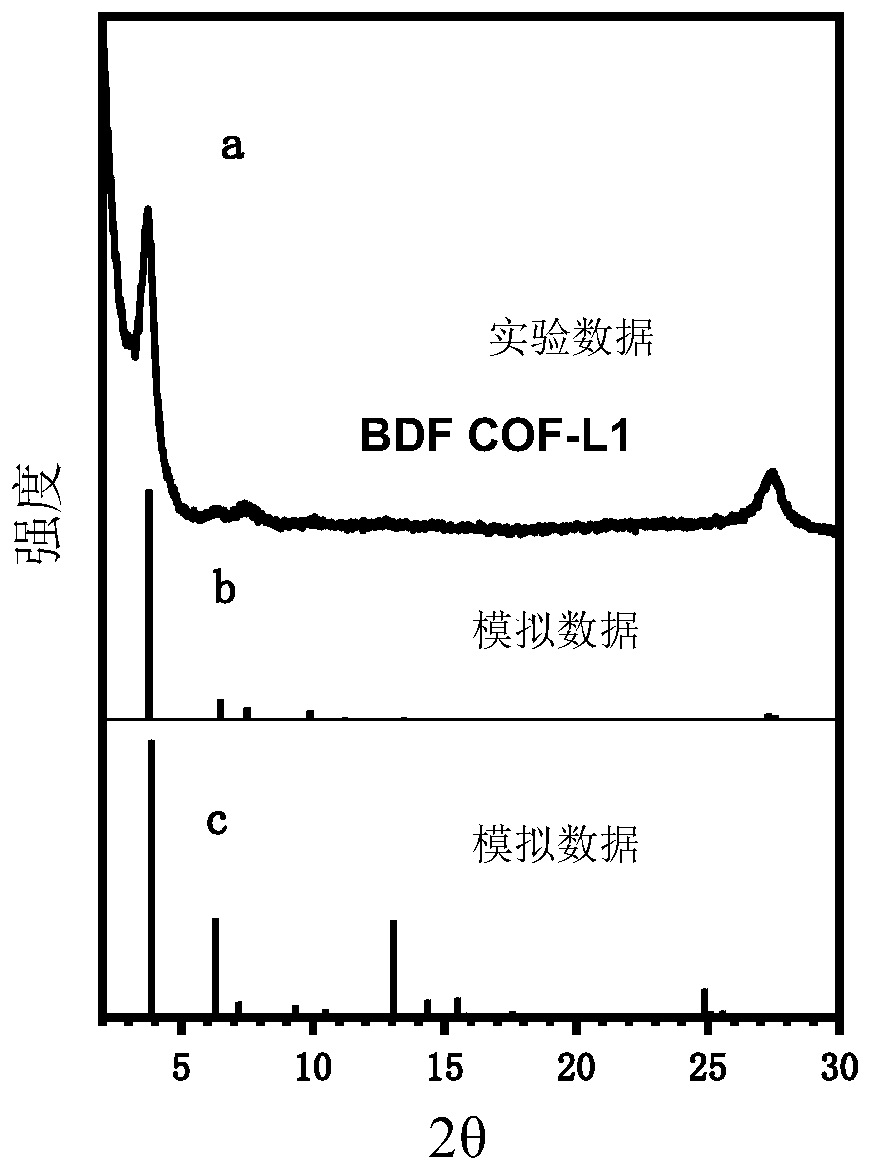
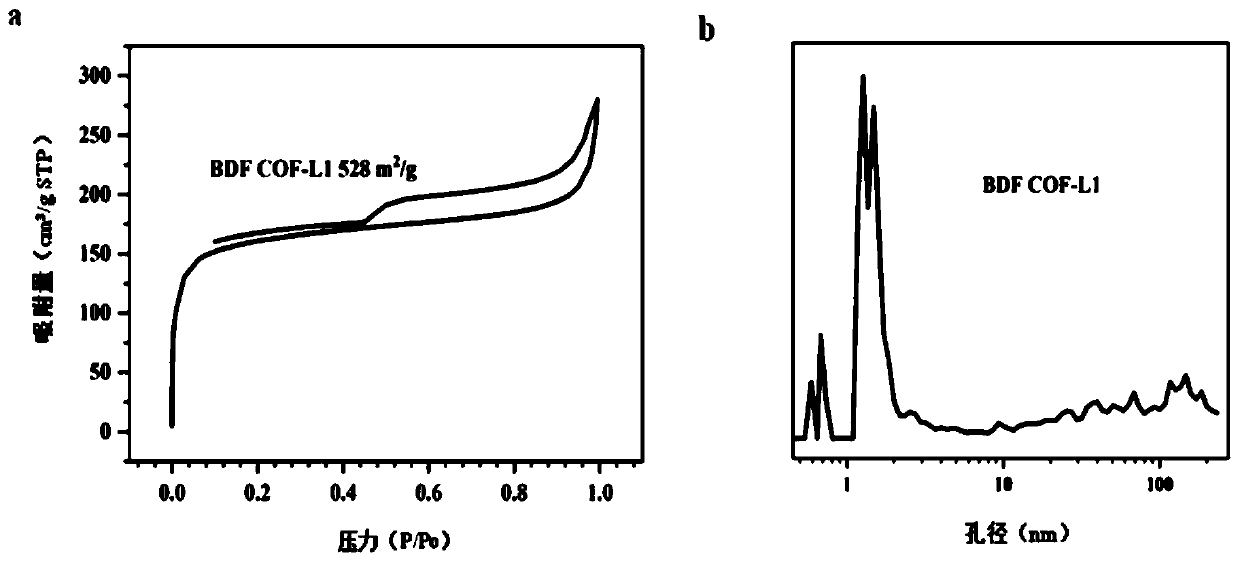
[0060] The synthesis method of the covalent organic framework material with benzofuran structure provided by the embodiment of the present invention comprises the following steps: 42 mg of 2,4,6-trihydroxy-trithenylaldehyde and 91 mg of 2,6-diaminobenzo[1 , 2-b:4,5-b'] Difuran-3,7-Dimethyl dicarboxylate was added to a heat-resistant glass tube, then 0.3ml 6mol / L acetic acid, 3ml o-dichlorobenzene and 3ml n-butyl Alcohol, secondly, the mixture was sonicated for five minutes, cycled through freezing-vacuumizing-thawing three times, sealed and placed in a blast oven, and reacted at 120°C for 72h. After the reaction was completed and cooled to room temperature naturally, the glass tube was opened, centrifuged, and washed 3 times with tetrahydrofuran, then placed in a vacuum oven and dried at 120°C for 10 hours to obtain a brown powder solid (named BDFCOF-L1).
Embodiment 2
[0062] The synthesis method of the covalent organic framework material with benzofuran structure provided in the embodiment of the present invention comprises the following steps: 42 mg of 2,4,6-trihydroxy-trithenylaldehyde and 99.6 mg of 2,6-diaminobenzo[ 1,2-b:4,5-b']difuran-3,7-diethyl carboxylate was added to a heat-resistant glass tube, and then 0.3ml of 6mol / L acetic acid, 3ml of o-dichlorobenzene and 3ml of n- Butanol, and secondly, the mixture was sonicated for five minutes, cycled through freezing-vacuumizing-thawing three times, sealed and placed in a blast oven, and reacted at 120°C for 72h. After the reaction was completed and cooled to room temperature naturally, the glass tube was opened, centrifuged, and washed 3 times with tetrahydrofuran, then placed in a vacuum oven and dried at 120°C for 10 hours to obtain a brown powder solid (named BDFCOF-L2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com