Three-dimensional covalent organic framework compound and preparation method and application thereof
A technology for covalent organic frameworks and compounds, applied in the field of three-dimensional covalent organic framework compounds and their preparation, can solve problems such as synthesis and preparation limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
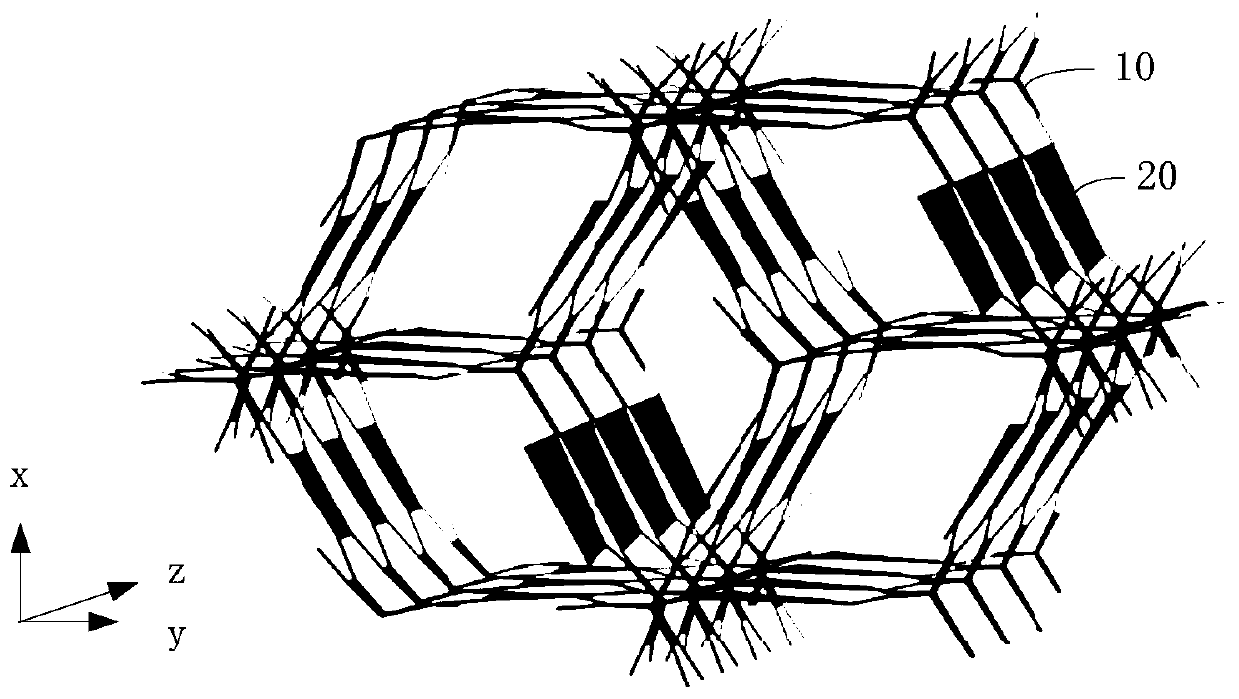
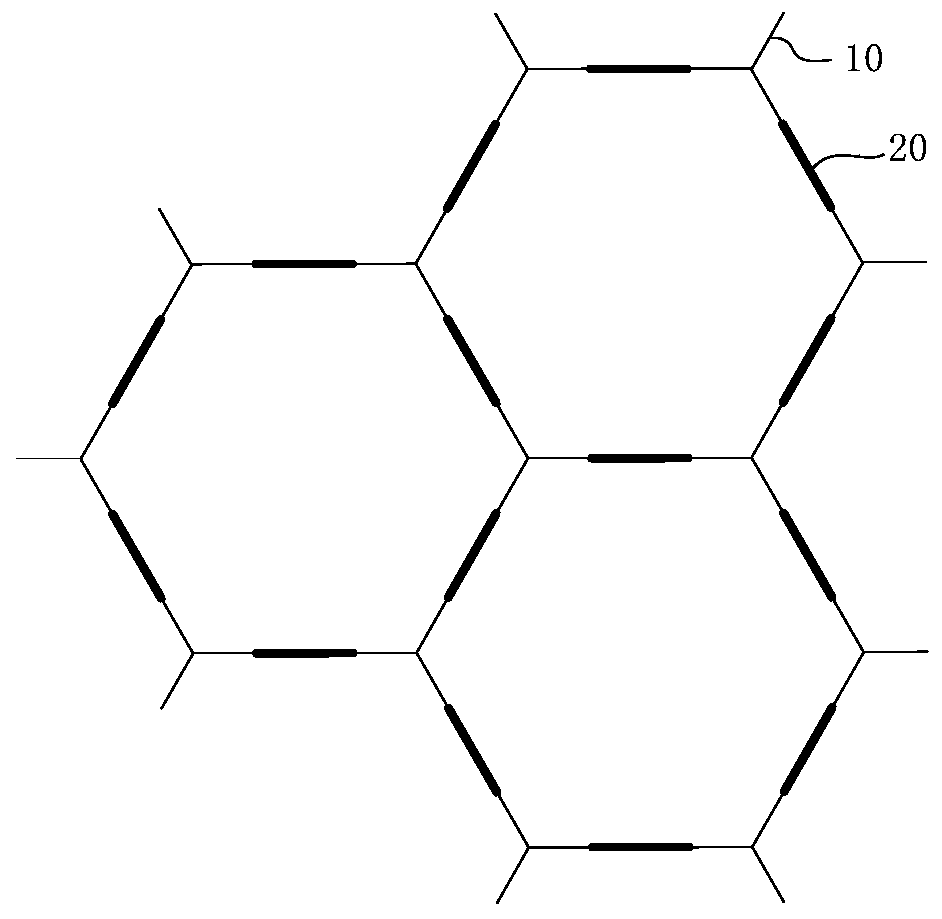
Image
Examples
preparation example Construction
[0060] The embodiment of the present invention also provides a preparation method of a three-dimensional covalent organic framework compound, comprising the following steps:
[0061] S1, put the triptycene compound shown in formula (5), the pyrene compound shown in formula (6) and the organic solvent together into a container, vacuumize and seal the container;
[0062] S2, heating the sealed container at 50°C to 200°C to make the triptycene compound react with the pyrene compound to form a solid precipitate;
[0063] S3, the precipitate is filtered out, soaked in an organic solvent, washed and dried, and the obtained solid product is the three-dimensional covalent organic framework compound.
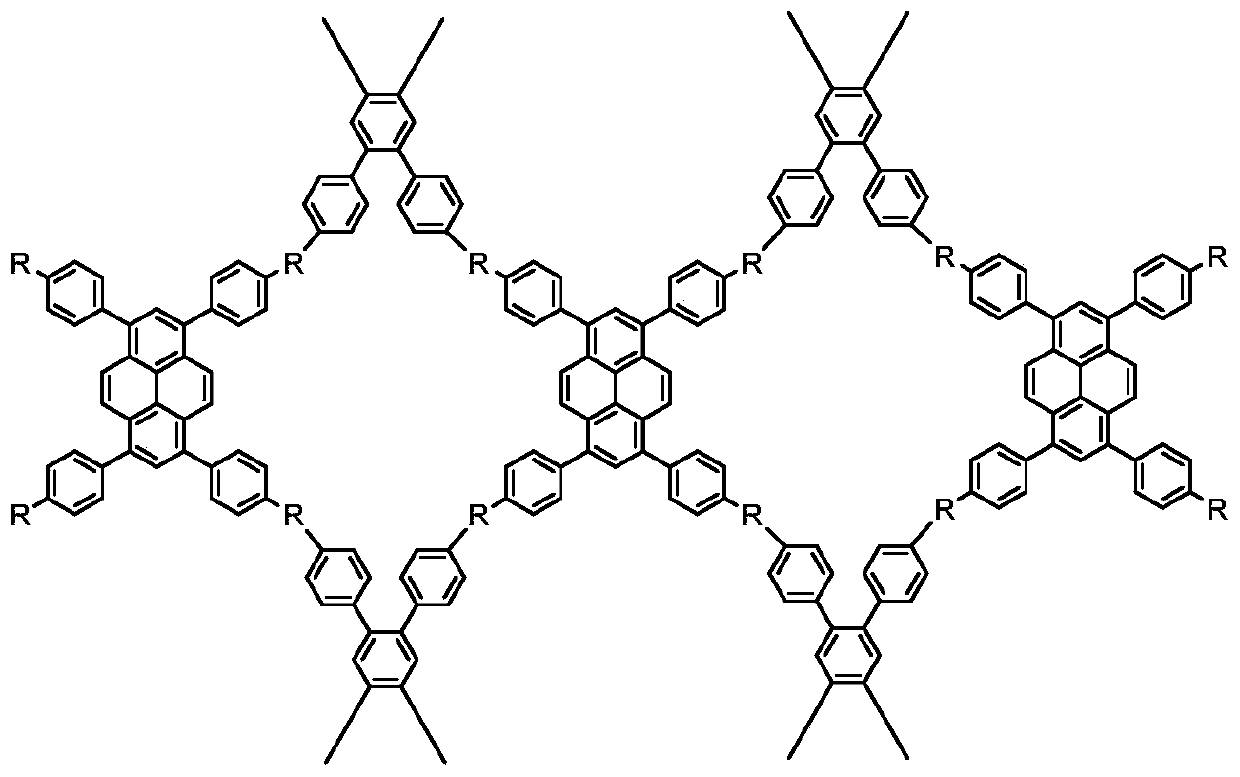
[0064]
[0065] R in formula (5) 1 and R in formula (6) 2 The reaction generates the linking group. For example, when the linking group is -C=N-, R 1 and R 2 One of them is an aldehyde group (-CHO), and the other is an amino group (-NH 2 ). When the linking group is -C=N-NH-, R...
Embodiment
[0078] (1) 2,3,6,7,14,15-hexa(4-formylphenyl)triptycene (2,3,6,7,14,15-hexa(4-formylphenyl)triptycene, HFPT) Synthesis
[0079]
[0080] According to the above formula, iron powder (0.22g, 3.94mmol) was added into a solution of triptycene (10.00g, 39.32mmol) in chloroform (150ml). Bromine (12 mL, 234.20 mmol) was added to the mixture at 0° C., and the mixture was stirred for 30 minutes, then heated to room temperature, and heated to reflux for 8 hours after a period of mild reaction. After the reaction, the mixture was cooled to room temperature, the solid iron powder catalyst was removed by filtration, and washed 3 times with water and saturated brine, the organic phase was evaporated, and the crude product was purified by silica gel column chromatography, and the eluent was dichloromethane to obtain 28 g of 2 ,3,6,7,14,15-hexabromotriptycene, yield 97.9%.
[0081]
[0082]Cesium carbonate (60.4g, 185.38mmol) and bis(triphenylphosphine)palladium(II) dichloride (1.4g, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com