Application of Akkermansia muciniphila in the preparation of probiotics for treating or preventing avian influenza virus infection
A technology of avian influenza virus and microecological preparations, which is applied in the direction of antiviral agents, medical raw materials derived from bacteria, and dispersion liquid delivery, which can solve problems such as changes in vaccine protection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation method of the probiotic preparation of anti-avian influenza virus infection:
[0029] 1. Preparation of pasteurized Akkermansia muciniphila preparations
[0030] Akkermansia muciniphila ATCC BAA-835. The lyophilized powder of the strain was resuspended with sterile PBS, and an appropriate amount of bacterial solution was picked with an inoculation loop to be rejuvenated by streaking on the BD company's brain heart infusion (BHI) agar supplemented with 0.25% mucin (Sigma) Incubate at 37°C for 48h under oxygen condition. Wash the bacterial colony with 1mL PBS, collect the bacterial suspension; take 50 μL of the bacterial suspension and spread it evenly on the BHI agar containing 0.25% mucin, and cultivate it under anaerobic conditions at 37°C for 48 hours; Rinse the bacterial lawn, collect the bacterial suspension, centrifuge at 4°C and 5000rpm for 10 minutes, discard the supernatant, resuspend the collected Akkermansia muciniphila pellet in PBS, count o...
Embodiment 2
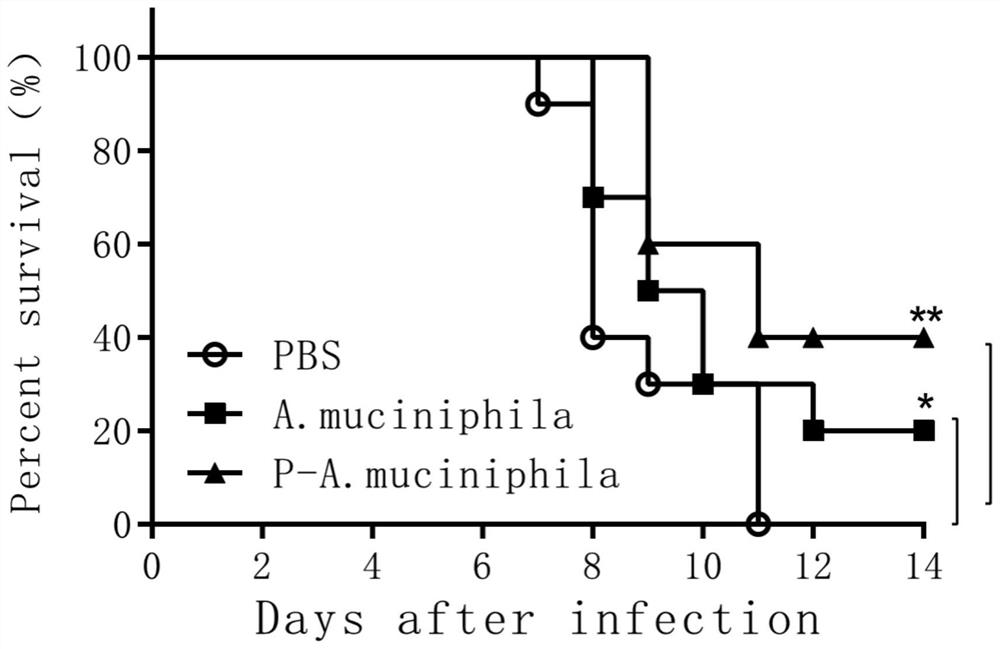
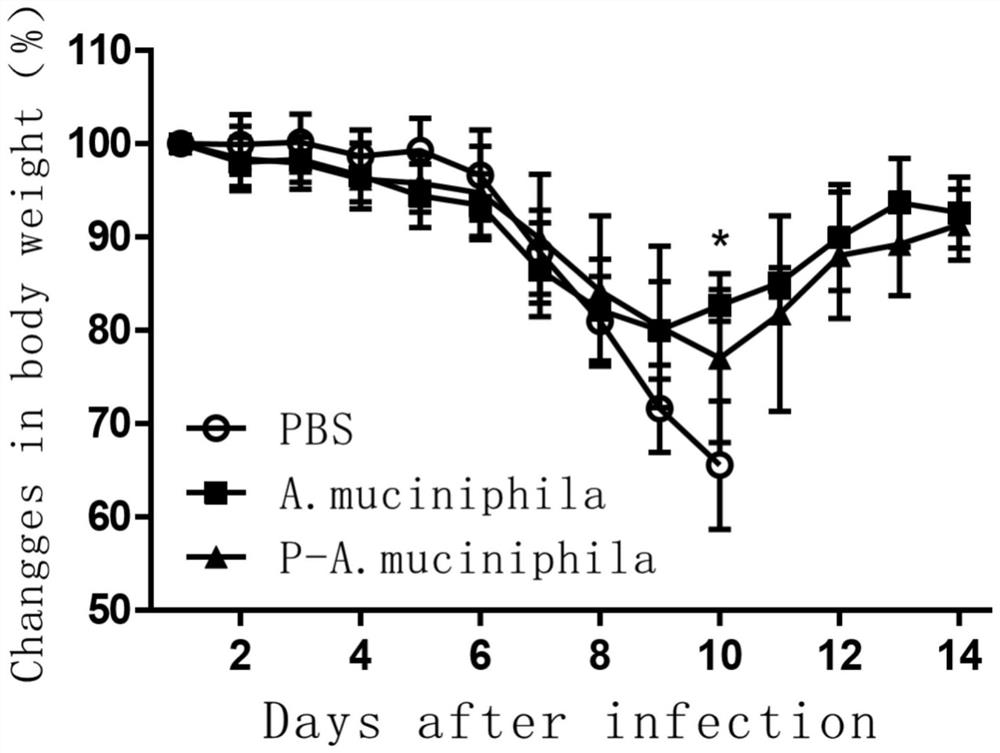
[0033] Effects of Akkermansia muciniphila ATCC BAA-835 on mortality and body weight of mice infected with H7N9 avian influenza virus
[0034] 1. Effects on Mortality and Body Weight of SPF Mice
[0035]The experimental animals were 8-week-old female C57BL / 6 specific pathogen-free (SPF) mice, a total of 30 were randomly divided into 3 groups, 10 in each group, and all animals were raised in a biosafety level 3 laboratory (ABSL3) . The first group is the control group fed with sterile PBS, 200ul per mouse; the second group is the group fed with live Akkermansia muciniphila (Akkermansia muciniphila) ATCC BAA-835 preparation, 200ul per mouse ; The third group was fed with pasteurized Akkermansia muciniphila (Akkermansia muciniphila) ATCC BAA-835 group, each mouse 200ul
[0036] Antibiotic treatment: Before the probiotic preparations were fed to the test mice, the mice were treated with a compound antibiotic solution, and the compound antibiotics were added to the sterile water t...
Embodiment 3
[0047] Effects of the inactivated probiotic preparation prepared in Example 1 on the influenza virus content in the lungs of mice infected with the H7N9 avian influenza virus, on the structure of the lungs of mice, and on the contents of cytokines in the lungs and blood.
[0048] The 36 SPF mice were randomly divided into 2 groups, 18 in each group. The first group was fed with sterile PBS control group, 200ul per mouse; the second group was fed with pasteurized mucin A For Akkermansia muciniphila ATCC BAA-835 group, 200ul per mouse;
[0049] As in Example 2, all SPF mice were raised in a biosafety level 3 laboratory (ABSL3), and underwent the same steps of antibiotic treatment, infusion of probiotic preparations and H7N9 influenza virus infection.
[0050] Lung tissue processing: Lung samples were dissected on day 0, day 3, and day 5 after infection, with 5 mice per group at each time point. Add 1ml PBS to each lung for homogenization, centrifuge at 8000rpm / min for 5min, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com