Synthesis method of N-diarylmethyl sulfonamide compound
A technology of diarylmethylsulfonamide and synthesis method, applied in the formation/introduction of sulfonyl/sulfinyl groups, preparation of sulfonamide, organic chemistry, etc., to achieve the effect of simple operation and economical raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the synthesis of N-(2,4,6-trimethoxyphenyl) benzylsulfonamide
[0030]
[0031] Benzaldehyde (106mg, 1.0mmol), 1 equivalent of p-toluenesulfonamide (171mg, 1.0mmol), FeCl 3 (16 mg, 0.1 mmol) and 1 equivalent of 1,3,5-trimethoxybenzene (168 mg, 1.0 mmol) were added to DCE (2 mL). The reaction was stirred at room temperature for 8 hours. After the reaction was completed, it was separated by column chromatography, and the eluting agent was a mixed solution of petroleum ether:ethyl acetate 2:1 to obtain 393 mg of a white solid product with a yield of 92%.
[0032] Its NMR data are as follows:
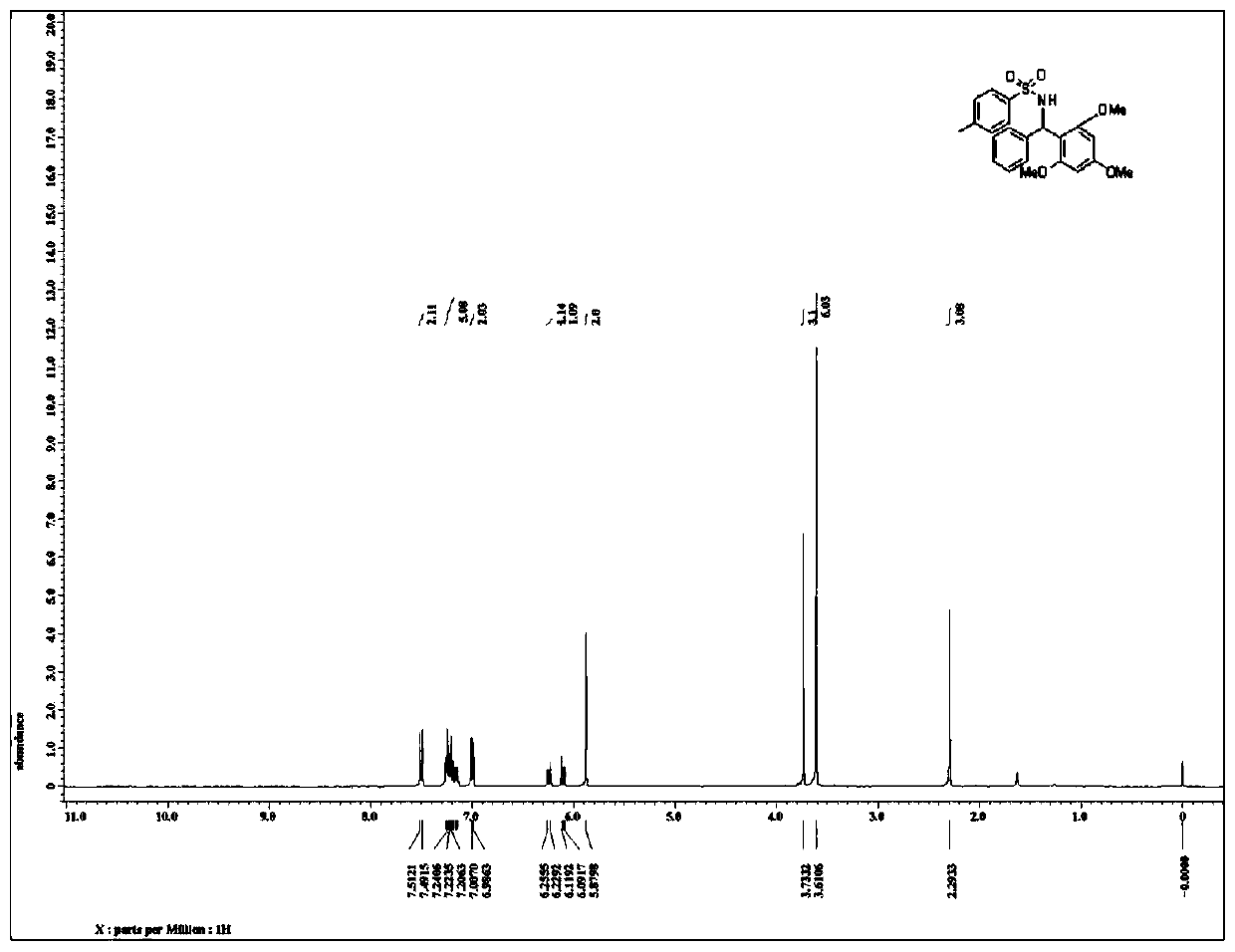
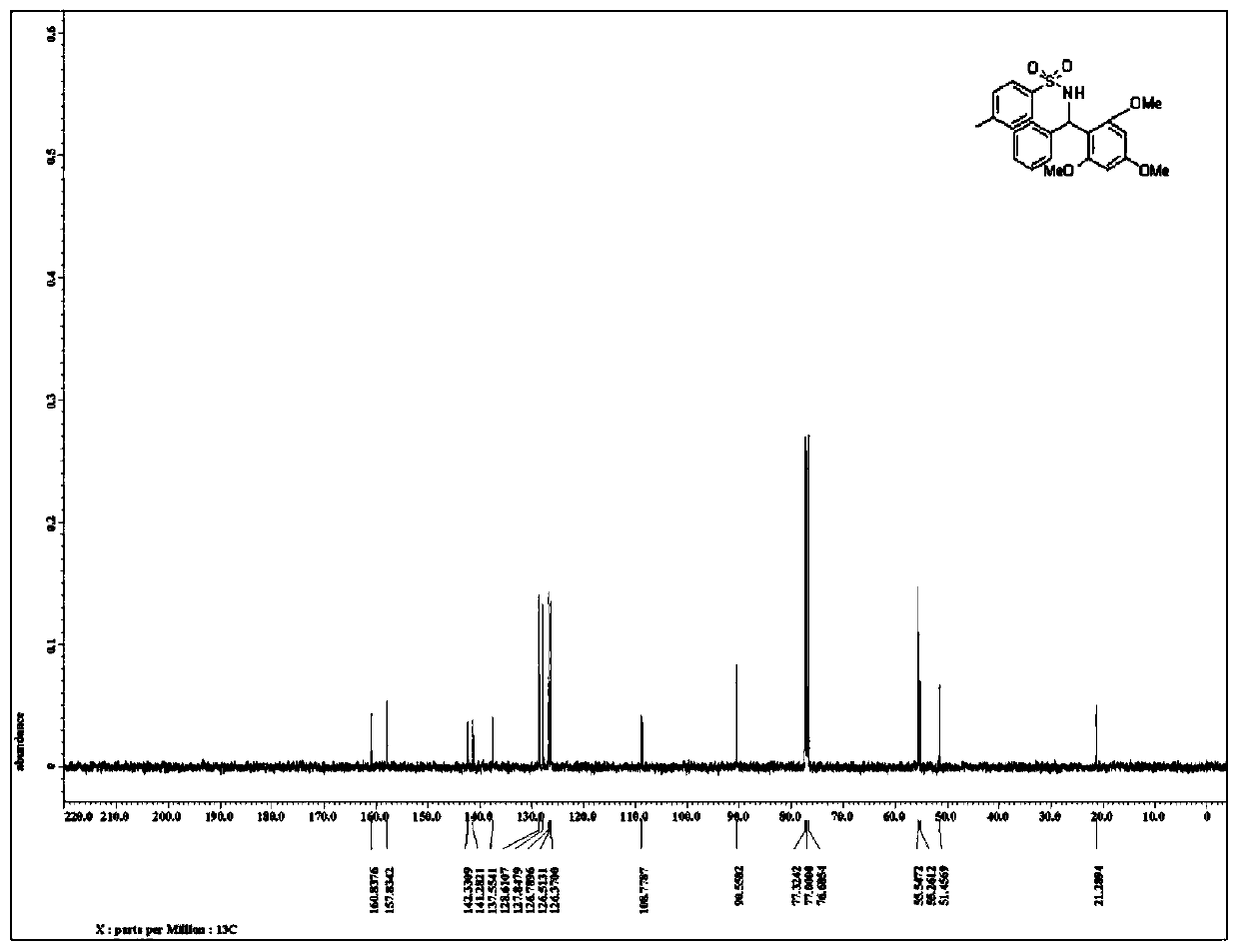
[0033] Such as figure 1 as shown, 1 H NMR (400MHz, CDCl 3 )7.50(d, J=8.4Hz, 2H), 7.26-7.15(m, 5H), 7.00(d, J=8.4Hz, 2H), 6.24(d, J=10.4Hz, 1H), 6.10(d, J=11.2Hz, 1H), 5.88(s, 2H), 3.72(s, 3H), 3.61(s, 6H), 2.30(s, 3H);
[0034] Such as figure 2 as shown, 13 C NMR (100MHz, CDCl 3 )160.8,157.8(2C),142.3,141.3,137.6,128.6(2C),127.8(2C),126.8,126.5(2C),126.4(2...
Embodiment 2
[0035] Embodiment 2: the synthesis of N-(2,4,6-trimethoxyphenyl) p-chlorobenzylsulfonamide
[0036]
[0037] P-chlorobenzaldehyde (140mg, 1.0mmol), 1 equivalent of p-toluenesulfonamide (171mg, 1.0mmol), FeCl 3 (16 mg, 0.1 mmol) and 1 equivalent of 1,3,5-trimethoxybenzene (168 mg, 1.0 mmol) were added to DCE (2 mL). The reaction was stirred at room temperature for 6 hours. After the reaction was completed, it was separated by column chromatography, and the eluent was a mixed solution of petroleum ether:ethyl acetate 2:1 to obtain 428 mg of a white solid product with a yield of 93%.
[0038] Its NMR data are as follows:
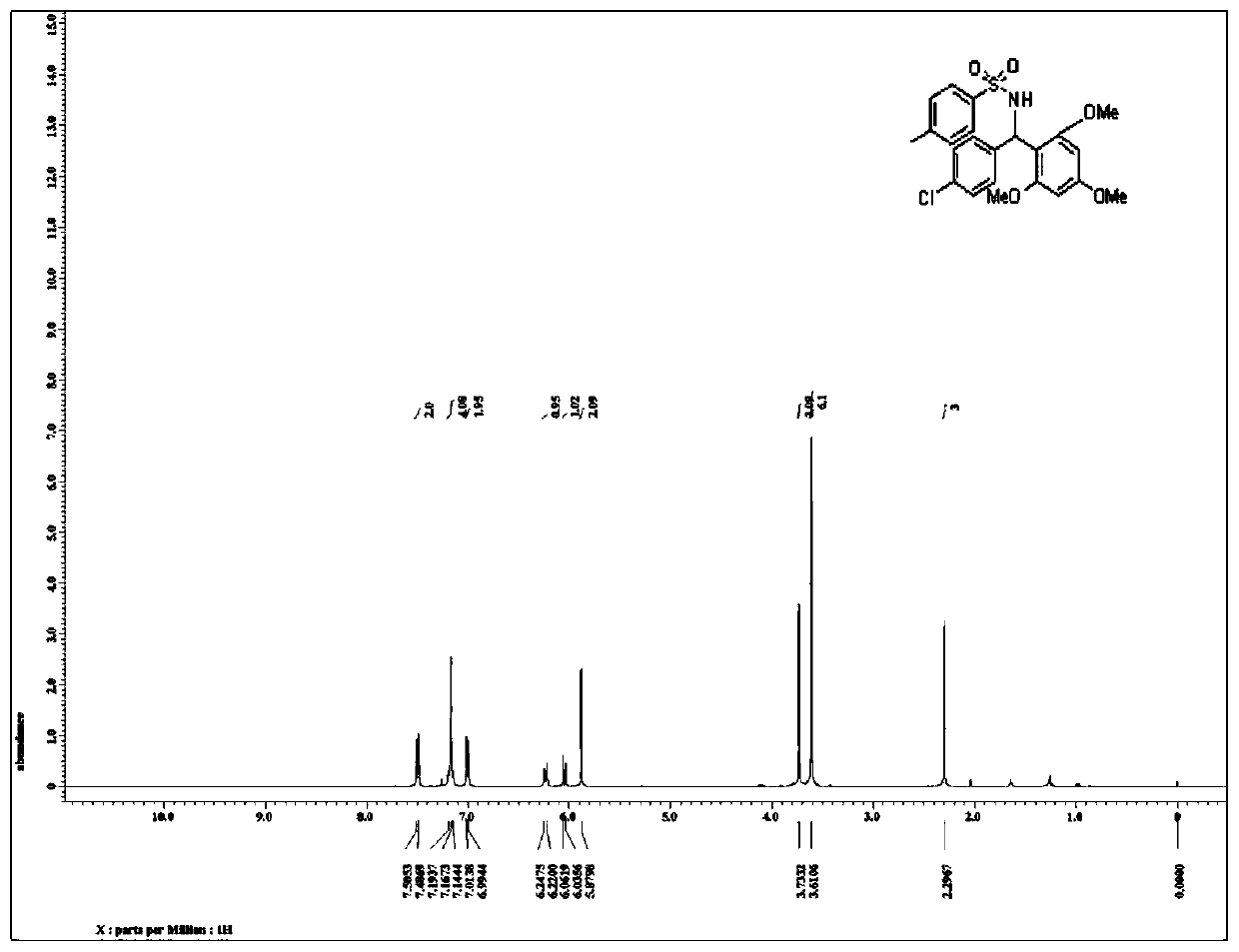
[0039] Such as image 3 as shown, 1 H NMR (400MHz, CDCl 3 )7.49(d, J=7.6Hz, 2H), 7.19-7.14(m, 4H), 7.00(d, J=7.6Hz, 2H), 6.23(d, J=10.8Hz, 1H), 6.06(d, J=10.4Hz, 1H), 5.88(s, 2H), 3.73(s, 3H), 3.61(s, 6H), 2.29(s, 3H);
[0040] Such as Figure 4 as shown, 13 C NMR (100MHz, CDCl 3 )2161.0,157.7(2C),142.5,140.0,137.4,131.2,128.7(2C),127.9(2C),127.8(2...
Embodiment 3
[0041] Embodiment 3: the synthesis of N-(2,4,6-trimethoxyphenyl) p-nitrobenzylsulfonamide
[0042]
[0043] P-chlorobenzaldehyde (140mg, 1.0mmol), 1 equivalent of p-toluenesulfonamide (171mg, 1.0mmol), FeCl 3 (16 mg, 0.1 mmol) and 1 equivalent of 1,3,5-trimethoxybenzene (168 mg, 1.0 mmol) were added to DCE (2 mL). The reaction was stirred at room temperature for 12 hours. After the reaction was completed, it was separated by column chromatography, and the eluting agent was a mixed solution of petroleum ether:ethyl acetate 1:1 to obtain 448 mg of a white solid product with a yield of 95%.
[0044] Its NMR data are as follows:
[0045] Such as Figure 5 as shown, 1 H NMR (300MHz, CDCl3 )8.03(d, J=9.2Hz, 2H), 7.54(d, J=8.4Hz, 2H), 7.44(d, J=8.8Hz, 2H), 7.04(d, J=8.0Hz, 2H), 6.48 (d,J=10.8Hz,1H),6.16(d,J=10.8Hz,1H),5.90(s,2H),3.73(s,3H),3.62(s,6H),2.31(s,3H) ;
[0046] Such as Figure 6 as shown, 13 C NMR (75MHz, CDCl 3 )161.3,157.5(2C),149.4,146.5,142.7,137.1,128.7(2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com