Application of 3-O-methyl quercetin to resisting oxidation or reducing blood sugar
A methyl quercetin, anti-oxidation technology, applied in the field of medicine, can solve problems such as side effects and oxidative damage, and achieve a significant effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
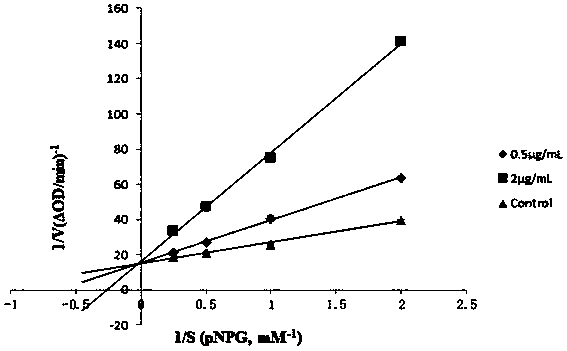
[0021] Inhibition of α-glucosidase activity by 3-O-methylquercetin in vitro
[0022] 1.1 Reagents: α-glucosidase (α-glucosidase, Sigma, 750U), 4-nitrophenyl-α-D-glucopyranoside (pNPG, TOKYO Chemica Industry Co., LTD), acarbose (Acarbose , TOKYO ChemicaIndustry Co., LTD), corosolic acid, Na 2 HPO 4 , NaH 2 PO 4 .
[0023] Comparator 1
[0024] Rhamnetin is a derivative of quercetin with the molecular formula C 16 h 12 o 7 ; Molecular weight: 316.26; CAS accession number: 90-19-7, structural formula:
[0025]
[0026] Comparator 2
[0027] Quercetin 3-rhamnoside is a kind of quercetin derivative with the molecular formula C 21 h 20 o 11 ; Molecular weight: 448.38; CAS accession number: 522-12-3, structural formula:
[0028]
[0029] Experimental equipment: microplate reader TECAN infinite M200 PRO (Teacan Group Ltd., Switzerland).
[0030] 1.2 Experimental steps
[0031] 1.2.1 Preparation of drug solution: first prepare 3-O-methyl quercetin, quercetin, acarb...
Embodiment 2
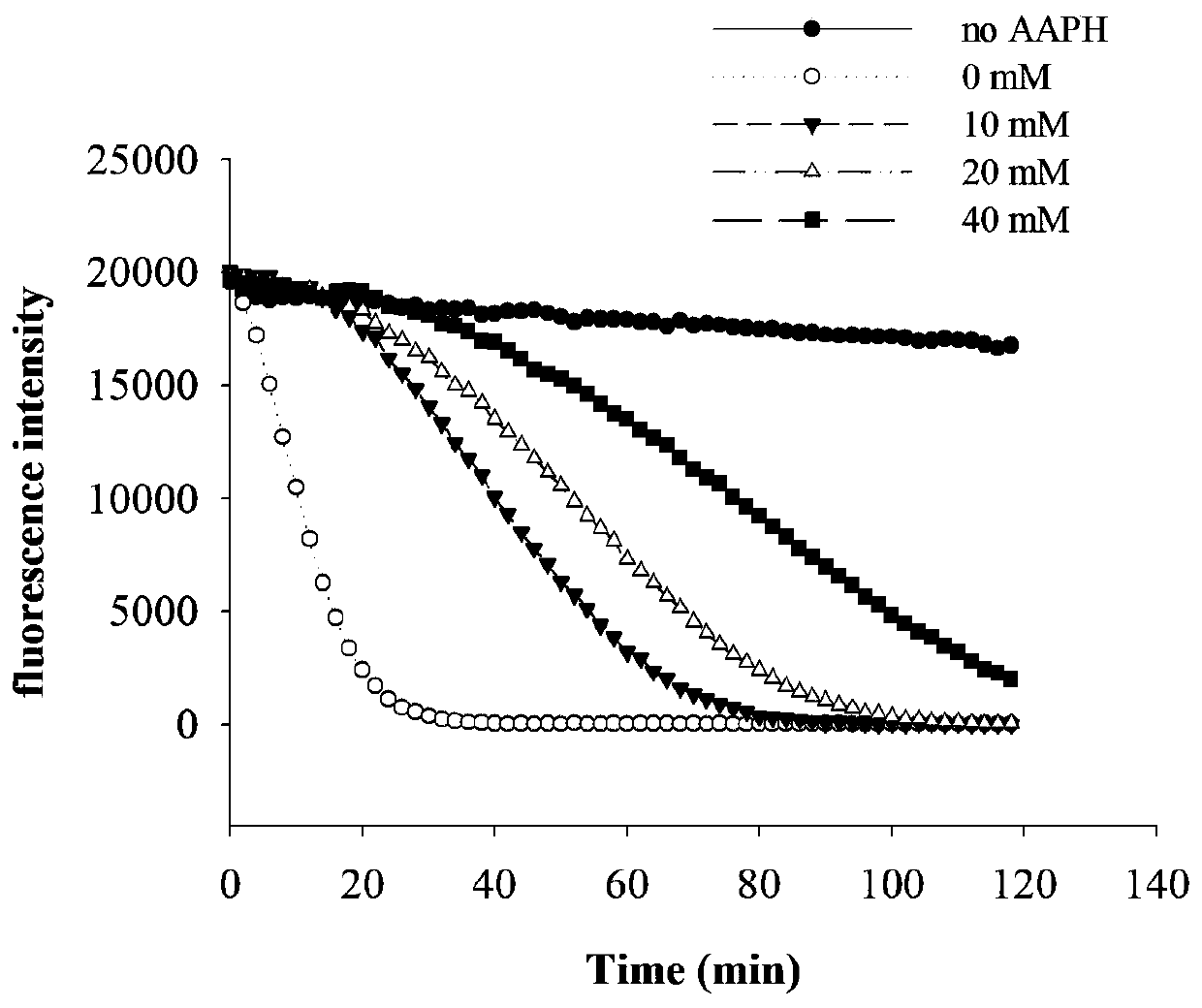
[0048] 3-O-Methylquercetin Antioxidative Ability Test in Vitro
[0049] 2.1 ORAC antioxidant experiment
[0050] 2.1.1 Reagents: vitamin E (Trolox), ABAP [2,2-Azobis (2-amidinopropane) dihydrochloride solution], DCFH–DA (2′,7′-Dichlorfluorescin diacetate), sodium fluorescein (Sodium Fluorescein), Na 2 HPO 4 , NaH 2 PO 4 . Experimental equipment: microplate reader TECAN infinite M200 PRO (Teacan Group Ltd., Switzerland).
[0051] 2.2 Experimental steps
[0052] 2.2.1 Preparation of drug solution: Prepare the sample to be tested from dimethyl sulfoxide (DMSO) into a 10 mg / mL mother solution; accurately weigh 20 mg of fluorescein sodium FL and dissolve it in 50 mL of PBS solution (pH=7.4), and prepare a concentration of 0.4mg / mL FLA solution, store in –20°C refrigerator; dissolve 200μL FLA solution in 50mL PBS solution to make FLB solution; dilute 500μL FLB solution to 50mL PBS solution to make FL working solution; weigh accurately Dissolve 2.5mg Trolox in 10mL PBS solutio...
Embodiment 3
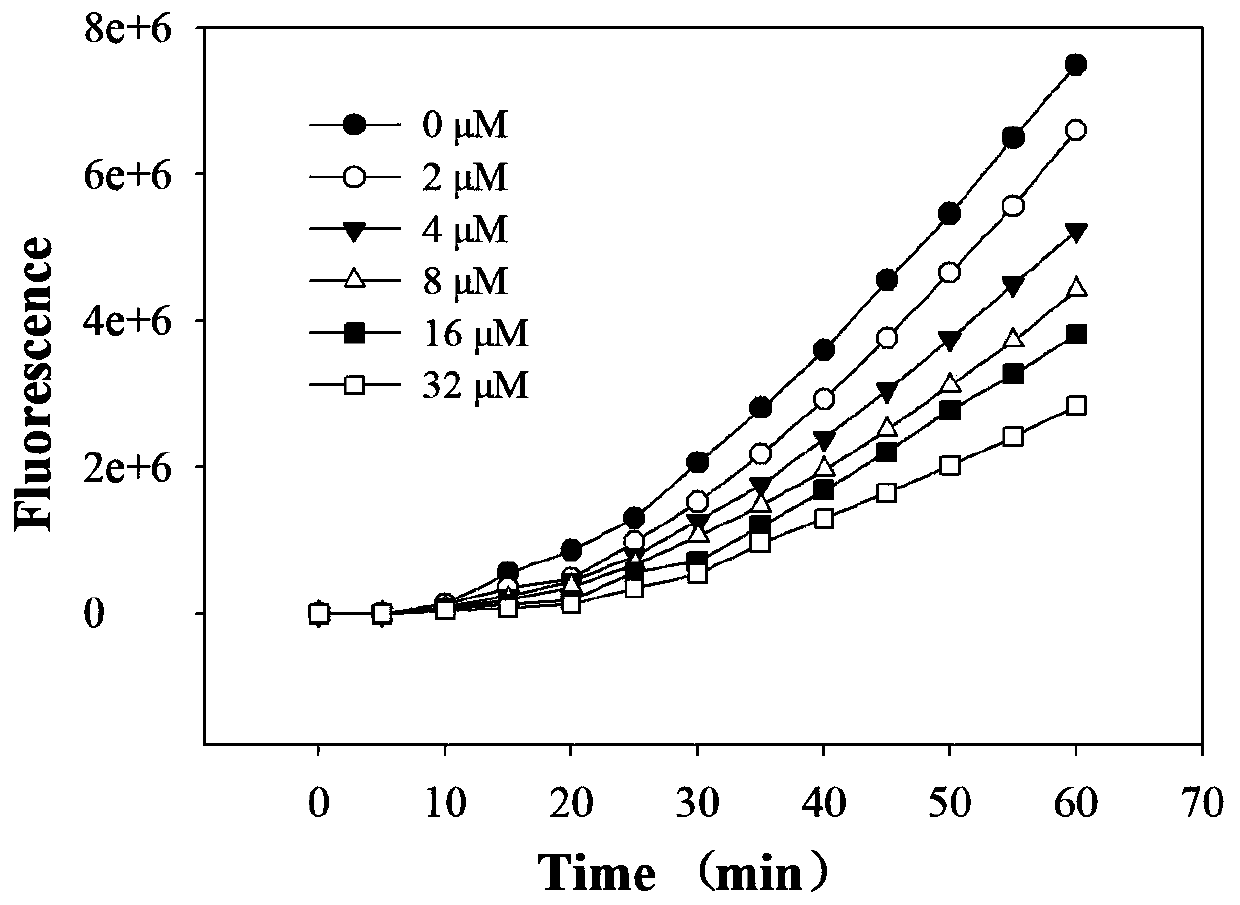
[0065] Intracellular Antioxidant (CAA) Experiment of 3-O-Methylquercetin
[0066] 3.1 Reagents: DCFH–DA (2′,7′-Dichlorfluorescin diacetate), sodium fluorescein (Sodium Fluorescein), ABAP [2,2-Azobis (2-amidinopropane) dihydrochloride solution], HepG2 liver cancer cells (Kunming Institute of Zoology, Chinese Academy of Sciences), MEM medium (Gibco), methanol (Tianjin Damao), L-glutamine (Gibco), Hanks balanced salt solution (HBSS), fetal bovine serum (FBS, Gibco), double antibody (Gibco), trypsin (Gibco) ). Experimental instrument: microplate reader TECAN infinite M200 PRO (Teacan Group Ltd., Swizerland)
[0067] 3.2 Experimental steps
[0068] 3.2.1 Principle: The CAA method is a detection of active oxygen by using the fluorescent probe DCFH-DA. DCFH-DA itself has no fluorescence, can freely pass through the cell membrane, enter the cell, and can be hydrolyzed by intracellular esterase DCFH is generated, and DCFH cannot pass through the cell membrane, so that the probe is e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com