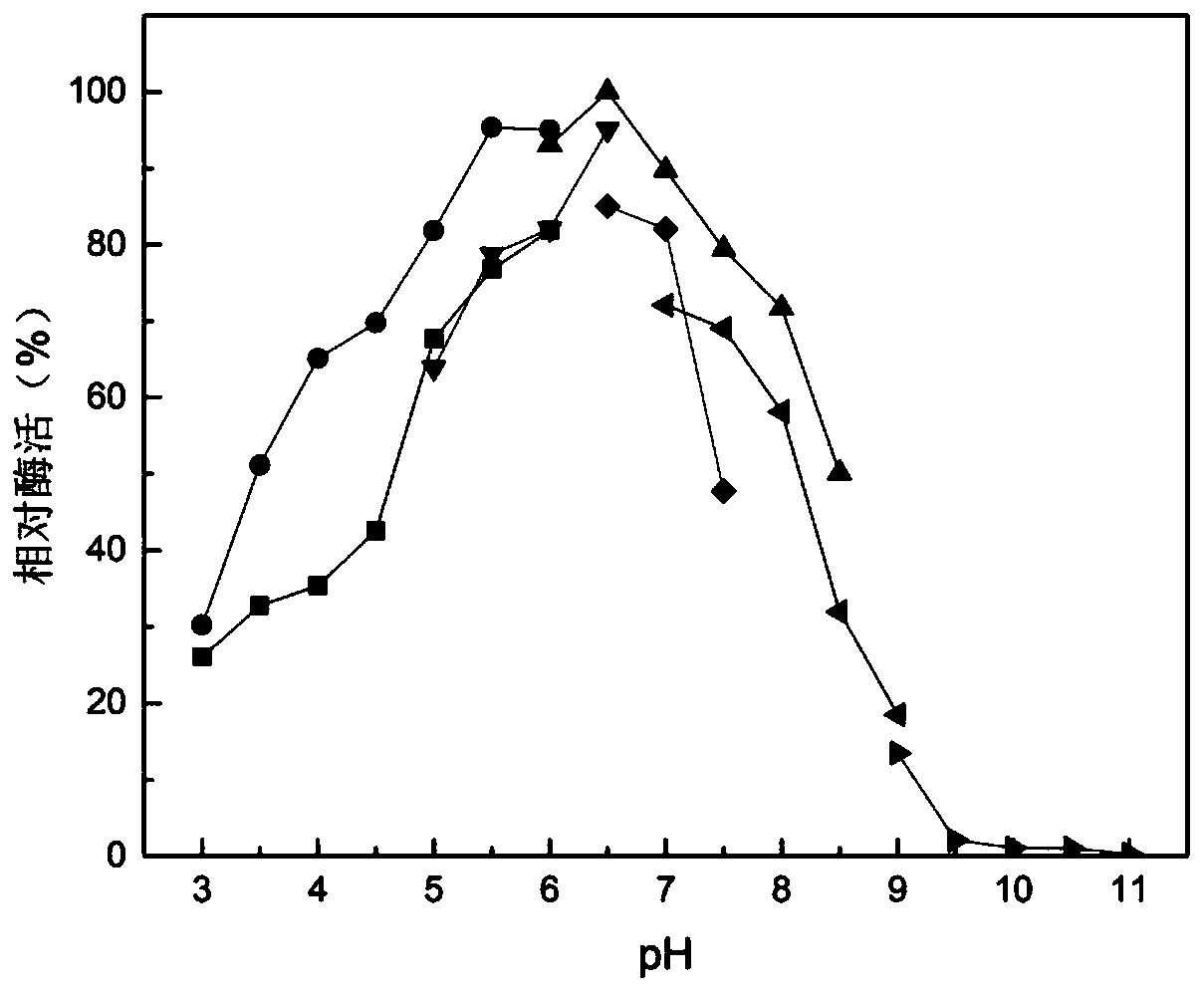
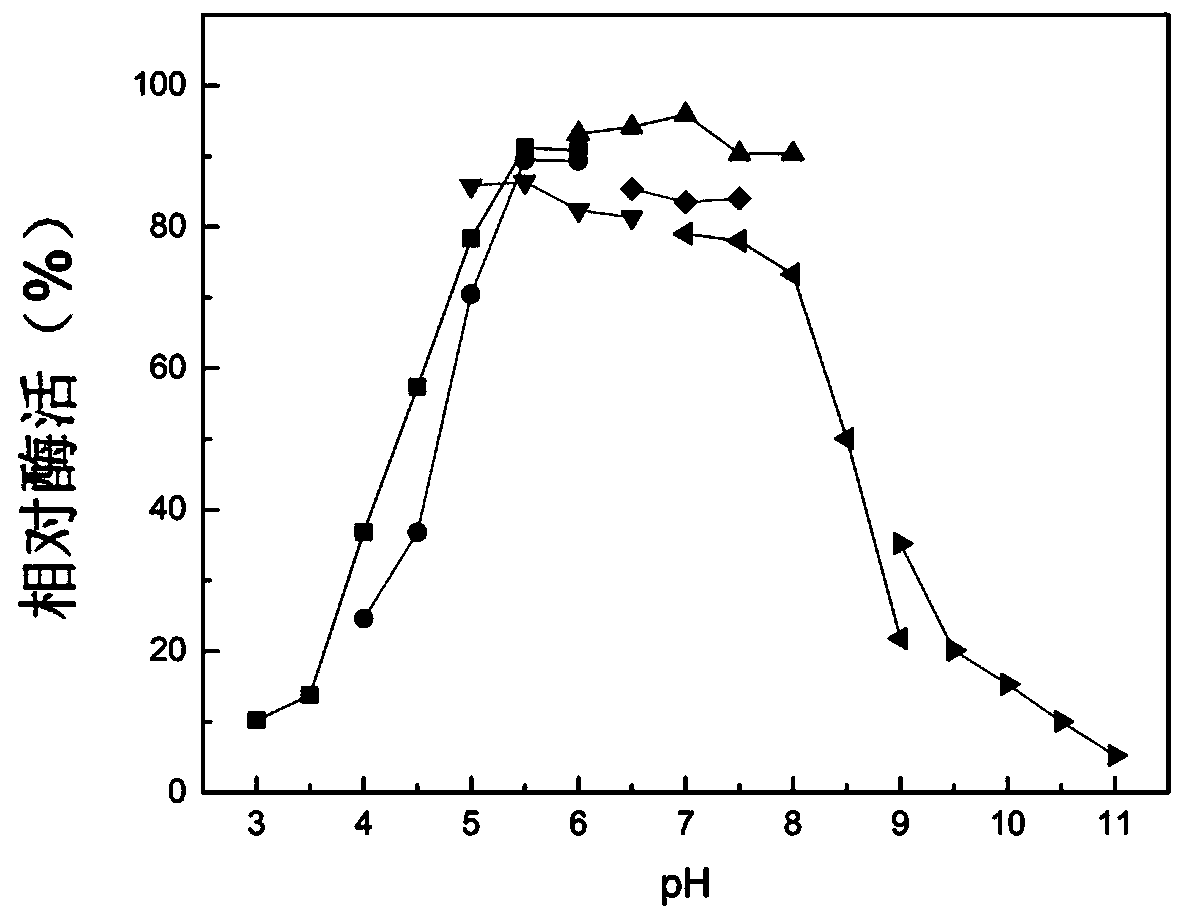
Microbulbifer arenaceous beta-galactosidase as well as encoding gene and application thereof
A technology of galactosidase and gene, which is applied in the field of Microvesicle β-galactosidase and its coding gene and its application, can solve the problems that Microvesicle β-galactosidase has not yet been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1, the cloning of Microvesicle sandum β-galactosidase and its coding gene
[0076] A large number of sequence analyzes and functional verifications were carried out on Microvesicle sandum BH1, from which a gene encoding β-galactosidase was cloned, with a full length of 2388bp, as shown in sequence 1 of the sequence list. The DNA molecule shown in sequence 1 of the sequence listing encodes the β-galactosidase shown in sequence 2, named MaBgal2A. MaBgal2A consists of 795 amino acids, of which the predicted 1st to 18th amino acids from the N-terminal are the signal peptide sequence.
Embodiment 2
[0077] Embodiment 2, the construction of engineered bacteria expressing Microvesicle sandum β-galactosidase
[0078] 1. Construction of vector for recombinant expression of Microvesicle sandum β-galactosidase
[0079] 1. Use the DNA fragment shown in the 55th-2388th position of the sequence 1 from the 5' end to replace the fragment between the NheI and XhoI restriction sites of the pET-28a (+) vector to obtain the recombinant expression vector pET-28a (+)-MaBgal2A (verified by sequencing).
[0080] The exogenously inserted DNA molecule is fused with part of the nucleotides on the carrier backbone to form a fusion gene shown in sequence 3 of the sequence listing, which encodes a fusion protein shown in sequence 4 of the sequence listing. In sequence 4 of the sequence listing, the 5th-10th position is a 6×His tag, and the 24th-800th position is MaBgal2A without signal peptide.
[0081] 2. Use the DNA fragment shown in the 55th-2388th position of the sequence 1 from the 5' end ...
Embodiment 3
[0089] Example 3, preparation and purification of Microvesicle sandum β-galactosidase and its enzymatic properties
[0090] 1. Preparation of recombinant protein MaBgal2A
[0091] 1. Inoculate the 4 kinds of recombinant bacteria prepared in Example 2 into LB liquid medium containing 50 μg / mL kanamycin respectively, and cultivate them with shaking at 37°C and 200 rpm until the bacterial liquid OD 600nm Reach between 0.6-0.8, add isopropyl-β-D-thiogalactopyranoside (IPTG) in the culture system, the concentration of IPTG in the culture system is 1mmol / L, 30 ℃, 200rpm induce and cultivate overnight, then The culture system was centrifuged at 11510g to collect bacterial precipitates, resuspended in buffer A and then sonicated (250W, 20min). After sonication, centrifuged at 11510g for 10min, and the supernatant was collected as the crude enzyme solution. After fragmentation, the target protein did not form inclusion bodies in Escherichia coli containing various expression vectors, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com