Curcumenol derivative containing triazole structure and application thereof in preparation of medicine for treating human colorectal cancer
A technology of triazole and curcumol, which is applied in the field of natural medicine and medicinal chemistry, can solve the problems of low anti-tumor activity and low water solubility, and achieve good biological activity, easy availability of raw materials, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
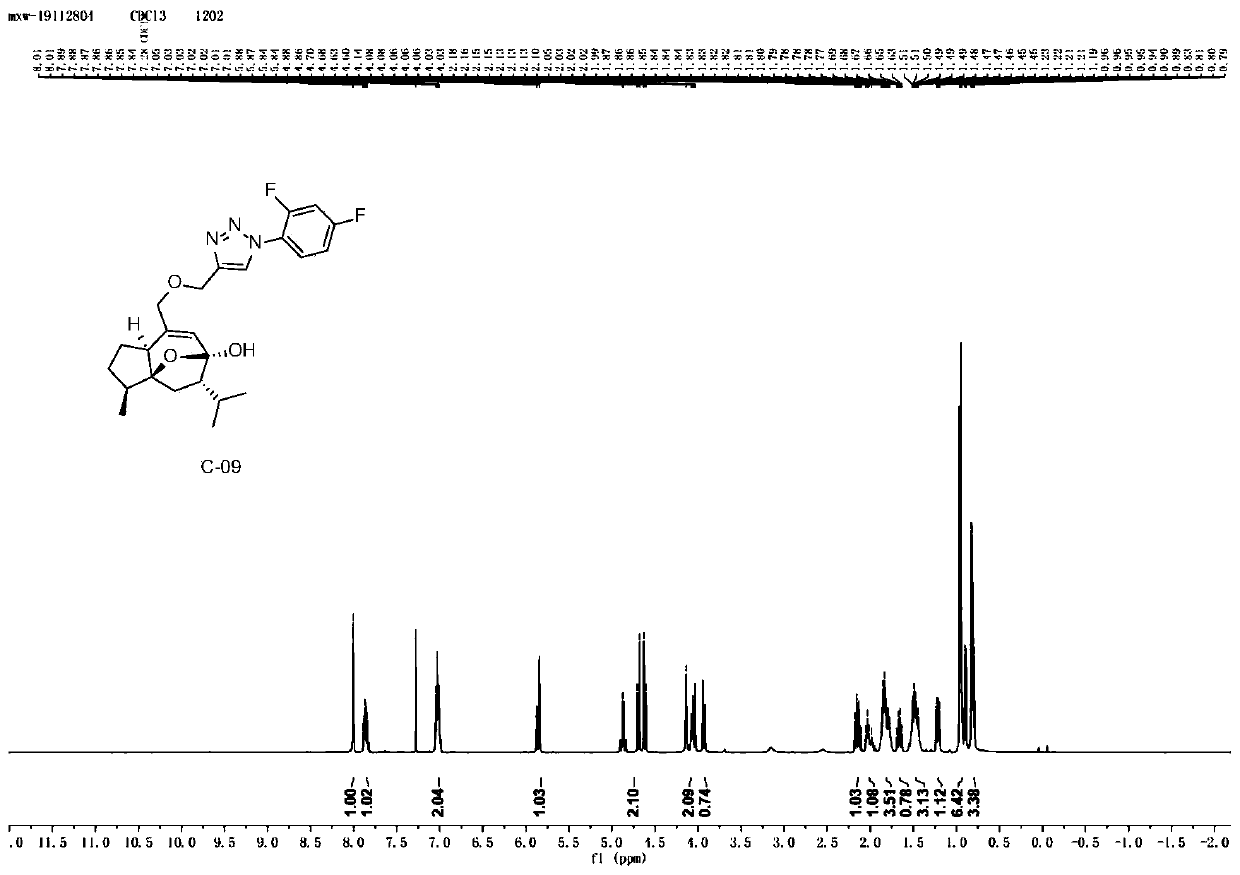
Examples
Embodiment 1
[0053] Example 1: Synthesis of Curcumol Derivative C-01 Containing Aryltriazole Structure
[0054]
[0055] Take 5.0g (21.15mmol) of curcumol, dissolve it in 50mL of dichloromethane, and add 7.30g (42.31mmol) of m-CPBA (m-chloroperoxybenzoic acid) in batches under the condition of ice-water bath. Stir at room temperature 25°C for 3h, check the reaction by TLC until the reaction is complete, concentrate the reaction solution, add saturated sodium bicarbonate to remove residual m-chloroperoxybenzoic acid, extract with ethyl acetate, combine the ethyl acetate phase, and saturated chlorine The ethyl acetate layer was washed three times with sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound 1-1 (4.87 g) as a light yellow oily product with a yield of 91.22%.
[0056] Take 2.0g (7.93mmol) of compound 1-1, dissolve it in 20mL of ethanol, heat to 70°C and stir, add 0.17g (4.25mmol) of sodium hydroxide, heat to r...
Embodiment 2
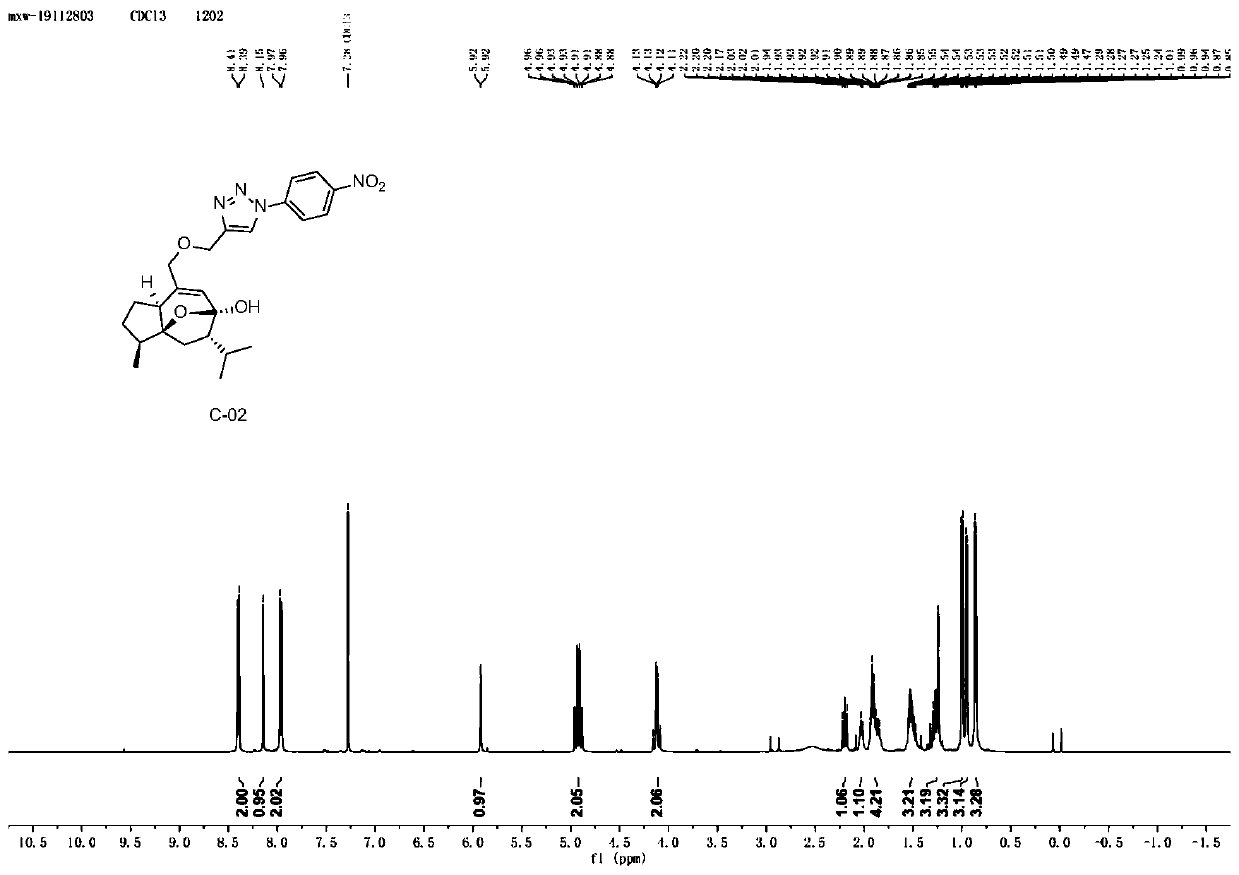
[0059] Example 2: Synthesis of Curcumol Derivative C-02 Containing Aryltriazole Structure
[0060]
[0061] Take 5.0g (21.15mmol) of curcumol, dissolve it in 50mL of dichloromethane, and add 7.30g (42.31mmol) of m-CPBA (m-chloroperoxybenzoic acid) in batches under the condition of ice-water bath. Stir at room temperature 25°C for 3h, check the reaction by TLC until the reaction is complete, concentrate the reaction solution, add saturated sodium bicarbonate to remove residual m-chloroperoxybenzoic acid, extract with ethyl acetate, combine the ethyl acetate phase, and saturated chlorine The ethyl acetate layer was washed three times with sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound 1-1 (4.87 g) as a light yellow oily product with a yield of 91.22%.
[0062] Take 2.0g (7.93mmol) of compound 1-1, dissolve it in 20mL of ethanol, heat to 70°C and stir, add 0.17g (4.25mmol) of sodium hydroxide, heat to r...
Embodiment 3
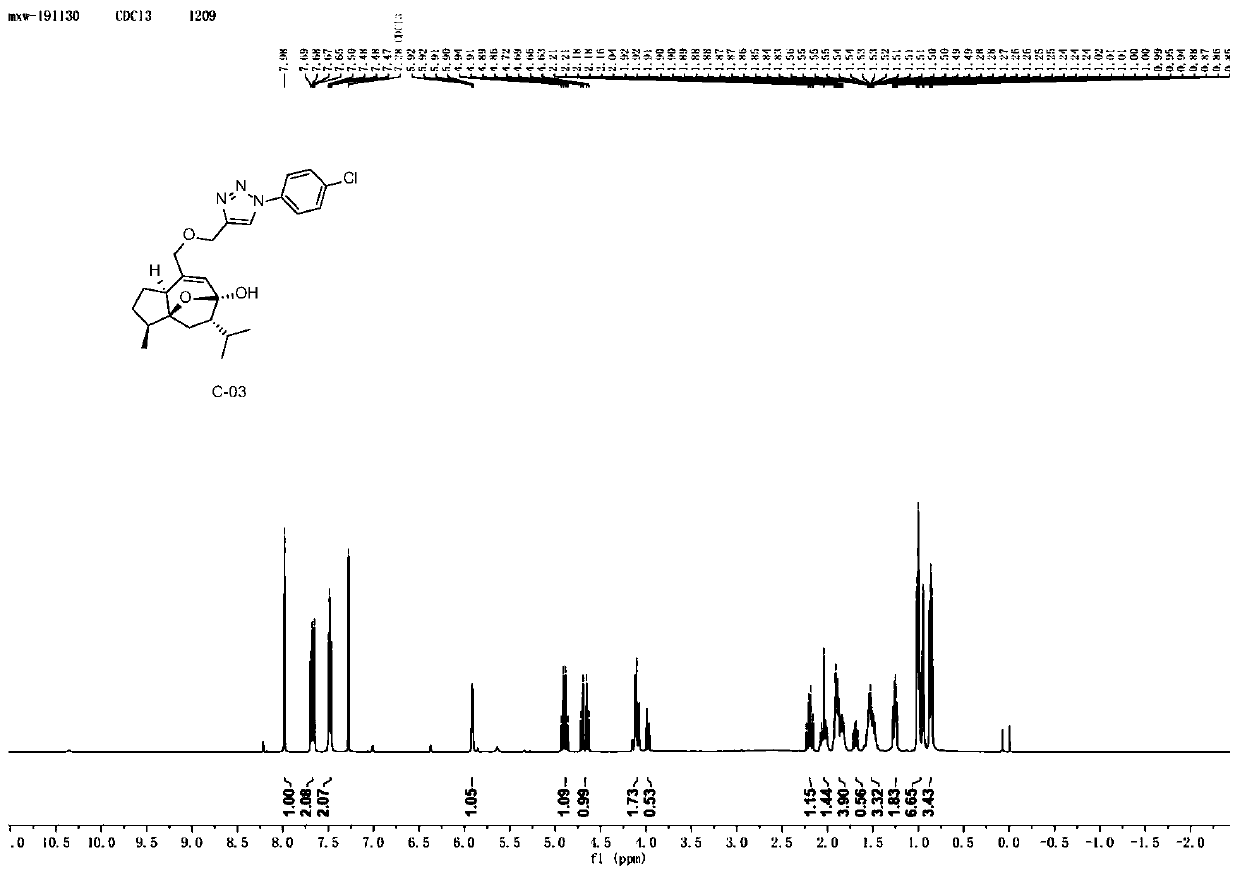
[0065] Example 3: Synthesis of Curcumol Derivative C-03 Containing Aryltriazole Structure
[0066]
[0067] Take 5.0g (21.15mmol) of curcumol, dissolve it in 50mL of dichloromethane, and add 7.30g (42.31mmol) of m-CPBA (m-chloroperoxybenzoic acid) in batches under the condition of ice-water bath. Stir at room temperature 25°C for 3h, check the reaction by TLC until the reaction is complete, concentrate the reaction solution, add saturated sodium bicarbonate to remove residual m-chloroperoxybenzoic acid, extract with ethyl acetate, combine the ethyl acetate phase, and saturated chlorine The ethyl acetate layer was washed three times with sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound 1-1 (4.87 g) as a light yellow oily product with a yield of 91.22%.
[0068] Take 2.0g (7.93mmol) of compound 1-1, dissolve it in 20mL of ethanol, heat to 70°C and stir, add 0.17g (4.25mmol) of sodium hydroxide, heat to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com