Recombinant mesenchymal stem cell, and preparation method and application thereof
A technology of stem cells and recombinant plasmids, applied in the field of genetic engineering, can solve the problems such as the inability to expand the scope of immunotherapy applications, the inability to efficiently achieve "cold" and "hot" tumor transformation, and the inability to efficiently change the immune microenvironment. To achieve the effect of changing the immune microenvironment, improving the efficiency, and increasing the content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0034] According to a preferred embodiment of the present invention, the mesenchymal stem cells are umbilical cord mesenchymal stem cells.
[0035] Wherein, the umbilical cord mesenchymal stem cells are derived from the isolated umbilical cord tissue, which is convenient to obtain, has a wide range of sources, is not subject to ethical debate and restrictions, and is not traumatic to the donor, and is not affected by the age of the donor. The culture is simple, the expansion is rapid, the immunogenicity is low, and there is no tumorigenicity, and it can be used as a cell carrier for gene therapy. Under specific induction conditions in vivo or in vitro, umbilical cord mesenchymal stem cells can differentiate into a variety of tissue cells, and they still have multidirectional differentiation potential after continuous subculture and cryopreservation. They are ideal seed cells for cell transplantation therapy.
[0036] According to a preferred embodiment of the present invention...
Embodiment 1
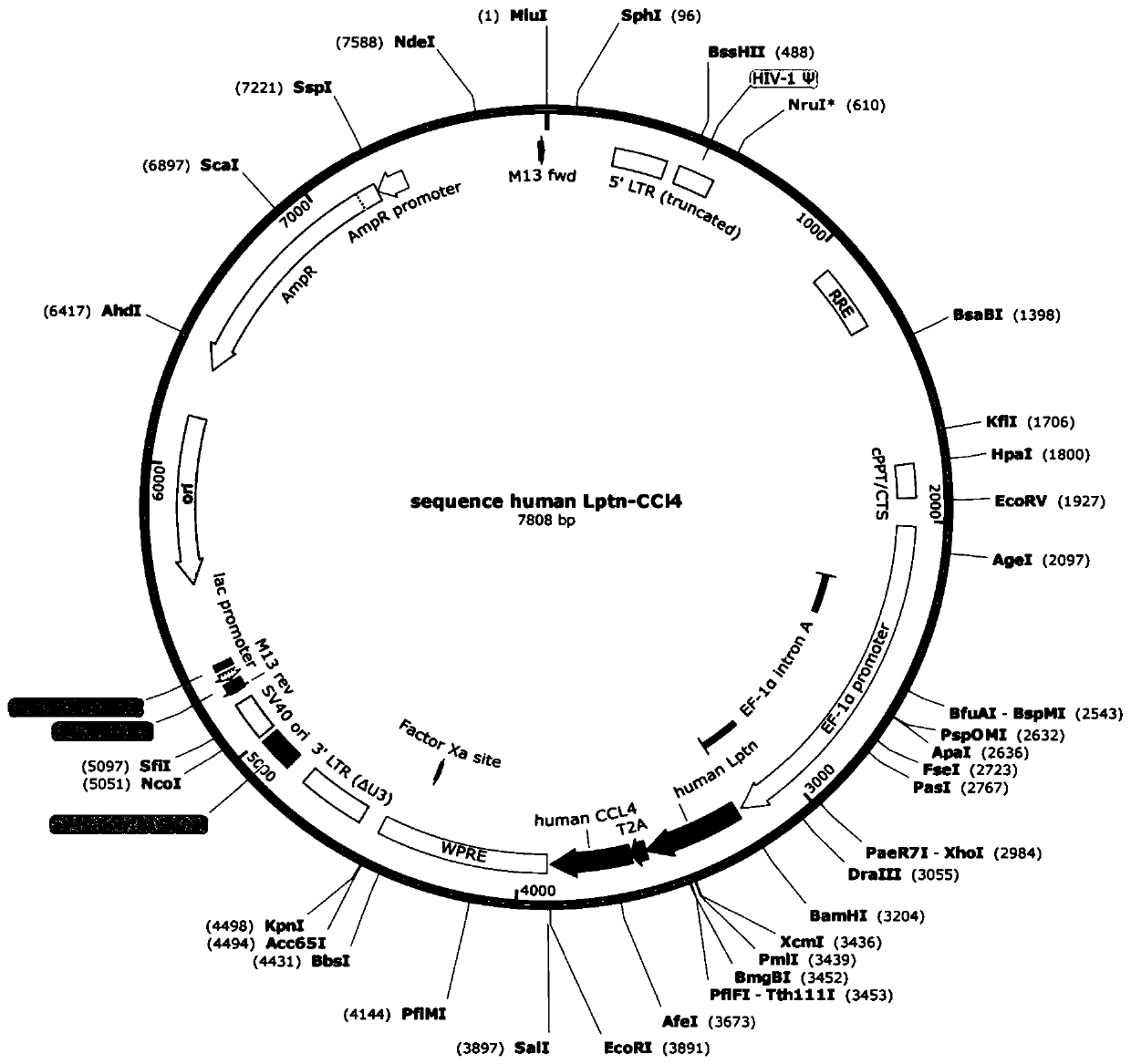
[0176] Embodiment 1 obtains Lptn-CCL4 fusion gene and recombinant plasmid thereof
[0177] (1) According to the human Lptn gene sequence in Genebank, the human Lptn gene was synthesized from the whole gene of Zhongmei Taihe Biotechnology (Beijing) Co., Ltd., its coding nucleotide sequence is the sequence shown in SEQ ID NO.5, and its amino acid The sequence is the sequence shown in SEQ ID NO.6.
[0178] According to the human CCL4 gene sequence in Genebank, the human CCL4 gene was synthesized from the whole gene of Zhongmei Taihe Biotechnology (Beijing) Co., Ltd., its coding nucleotide sequence is the sequence shown in SEQ ID NO.7, and its amino acid sequence is SEQ ID NO.7 The sequence shown in ID NO.8.
[0179] The above-mentioned human Lptn gene and human CCL4 gene were synthesized by Zhongmei Taihe Biotechnology (Beijing) Co., Ltd. into a DNA molecule encoding human Lptn-CCL4. The T2A sequence (the coding nucleotide sequence is the sequence shown in SEQ ID NO.3, and th...
Embodiment 2
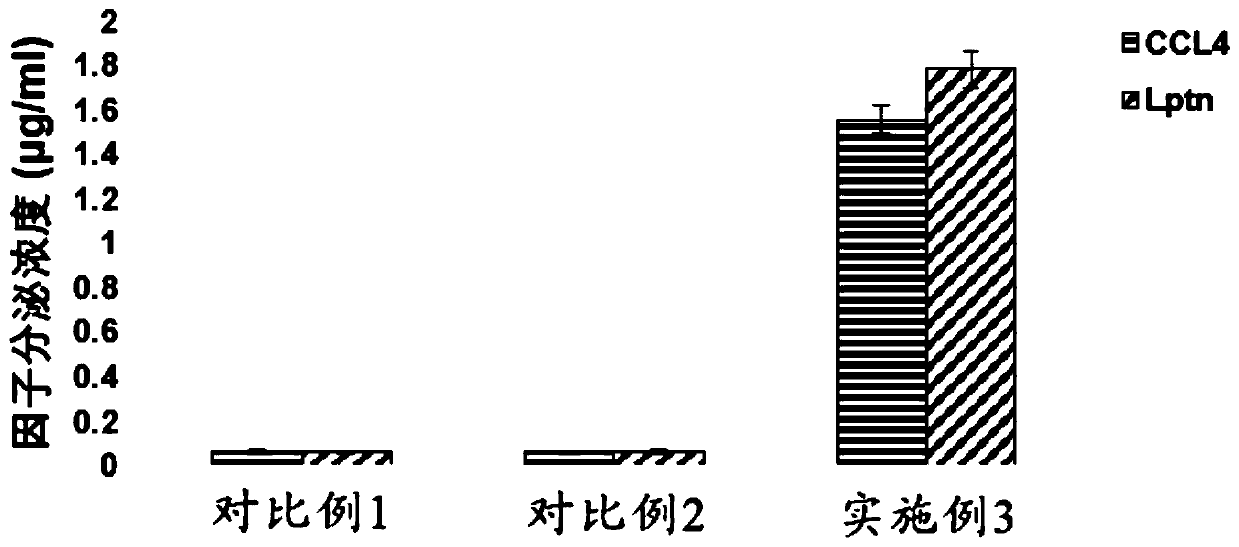
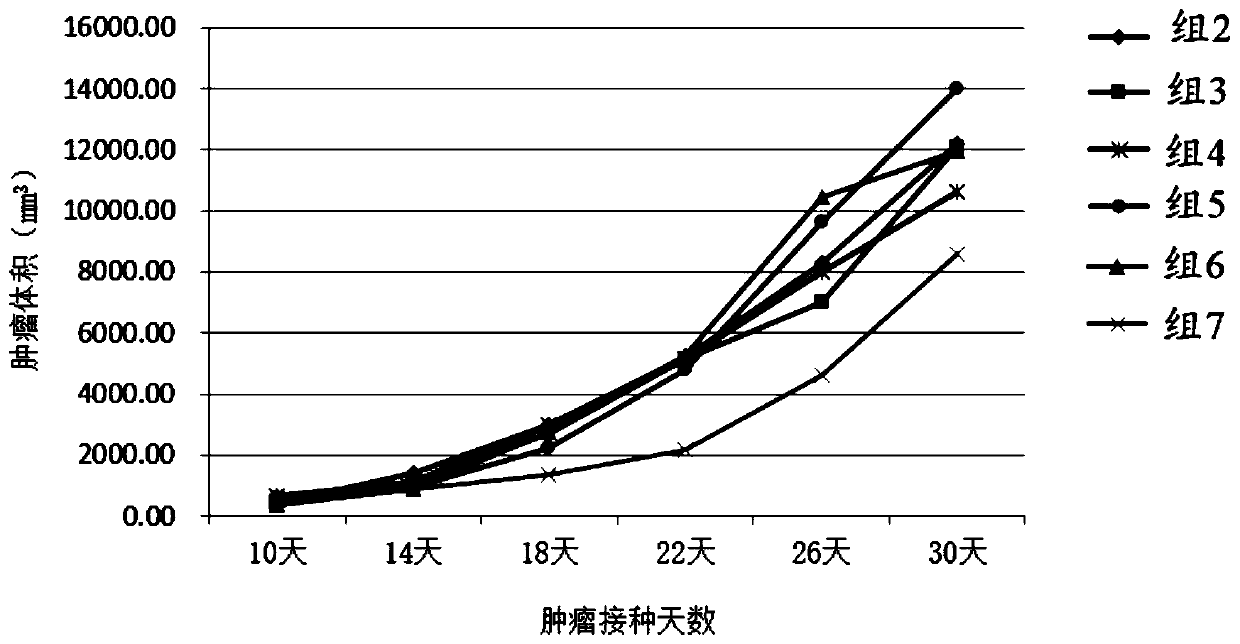
[0182] Example 2 Preparation of recombinant lentivirus carrying Lptn-CCL4
[0183] (1) Take out a tube of frozen 293T cells (purchased from ATCC) from liquid nitrogen and quickly put it in a 37°C water bath until the ice cubes disappear, add dropwise to a 15ml centrifuge tube containing 5ml preheated medium, and centrifuge at 1200rpm for 3min , Discard the supernatant, resuspend the cells in 293T medium (10% FBS + 1mM sodium pyruvate + 2mM glutamine + 1% non-essential amino acids + DMEM) and inoculate them in a 150mm culture dish at 37°C, 5% CO 2 Cultivate in saturated humidity. During the culture process, when the confluence of the cells reaches more than 90%, carry out subculture, discard the old medium, add 5ml sterilized PBS solution, shake gently, discard the PBS solution after washing the cells, and add 2ml 0.25% Trypsin- EDTA digestion solution, digest for 1-2min until the cells are completely digested; add serum-containing medium to stop the digestion, and centrifug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com