Tirofiban hydrochloride intermediate and preparation method of tirofiban hydrochloride
A technology of tirofiban and an intermediate, which is applied in the field of medicine, can solve the problems of easy racemization, no report on the preparation method of tirofiban hydrochloride, racemization of chiral center, etc., and achieves stable quality and guarantees clinical efficacy. and drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] The preparation of embodiment 1 tirofiban hydrochloride intermediate N-butanesulfonyl-O-[4-(4-pyridyl)-butyl]-L-tyrosine
[0104] A In dimethylsulfoxide (300ml), add 50g (0.166mol) of N-(butanesulfonyl)-L-tyrosine, 34.3g (0.166mol) of 4-(4-pyridyl) butyl chloride hydrochloride , 1.00eq), catalyst sodium iodide 2.0g, sodium hydroxide solution (0.631mol, 3.8eq) was added dropwise under stirring, after the addition was completed, the temperature was raised to 65-75°C, and the temperature was controlled at 65-75°C for 12h.
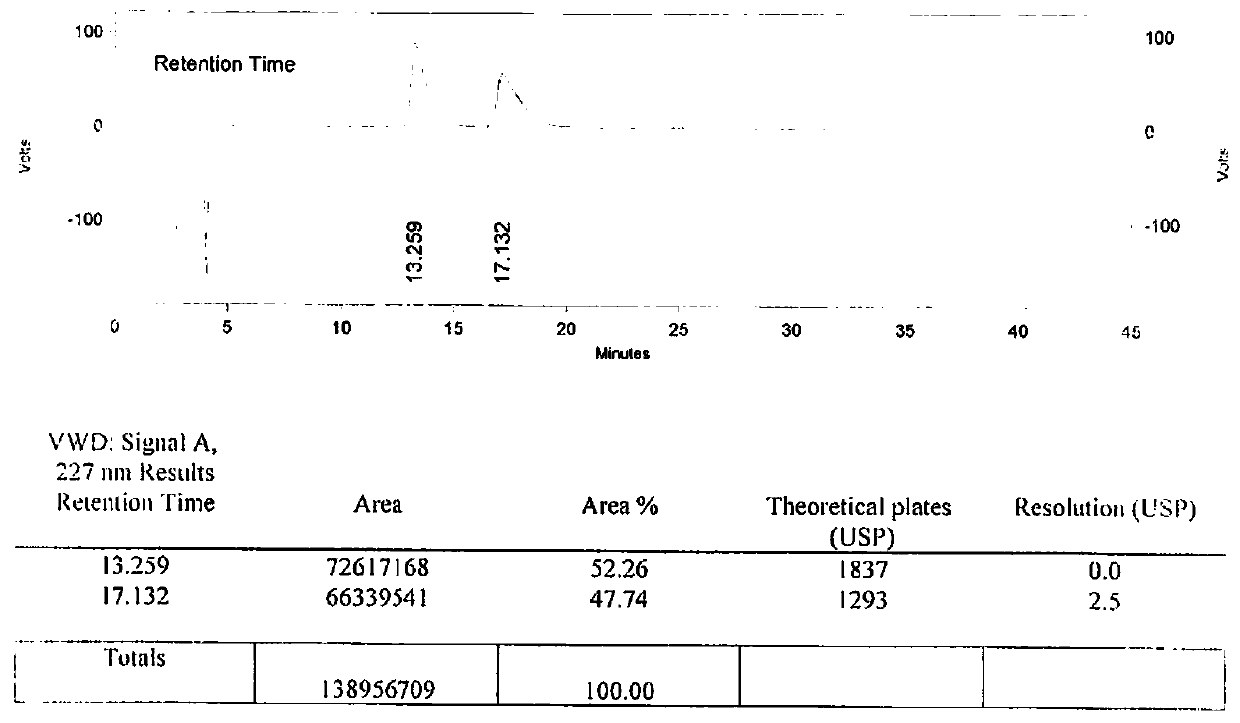
[0105] B After the reaction is completed, add 1.5L of purified water and 1L of dichloromethane, adjust the pH value to 5.5 with dilute hydrochloric acid, stand still and separate the phases, take the organic phase (dichloromethane phase), and concentrate under reduced pressure to obtain off-white solid N-butanesulfonate Acyl-O-[4-(4-pyridyl)-butyl]-L-tyrosine. The weight is 45.55g, the molar yield is 63.14%, and the isomer content is 0.48%. See the li...
Embodiment 2
[0107] The preparation of embodiment 2 tirofiban hydrochloride intermediate N-butanesulfonyl-O-[4-(4-pyridyl)-butyl]-L-tyrosine
[0108] A In dimethylsulfoxide (450ml), add 50g (0.166mol) of N-(butanesulfonyl)-L-tyrosine, 44.5g (0.216mol) of 4-(4-pyridyl) butyl chloride hydrochloride , 1.30eq), catalyst potassium iodide 2.5g, sodium hydroxide solution (0.664mol, 4eq) was added dropwise under stirring, after the addition was completed, the temperature was raised to 65-75°C, and the temperature was controlled at 65-75°C for 13h.
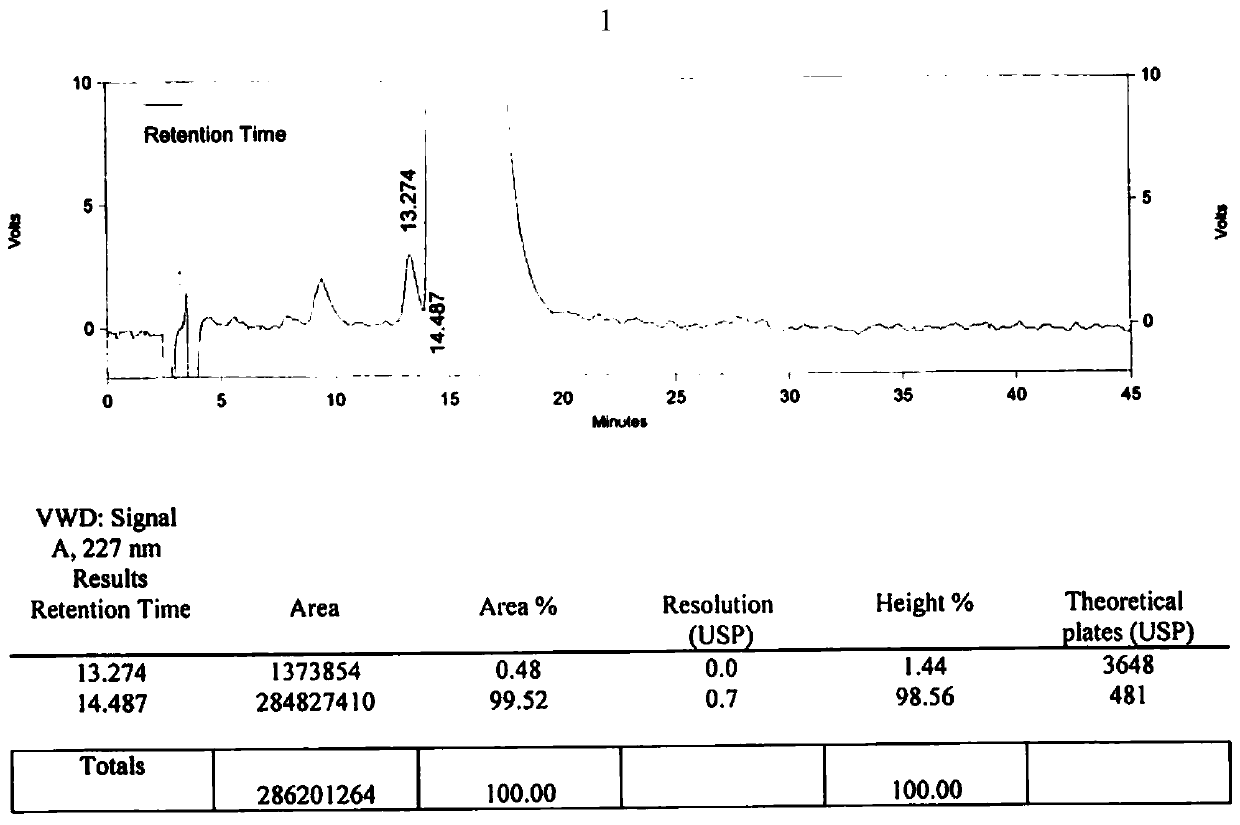
[0109] B After the reaction is completed, add 2.5L of purified water and 1.5L of dichloromethane, adjust the pH value to 6 with dilute hydrochloric acid, let stand to separate the phases, take the organic phase (dichloromethane phase), and concentrate under reduced pressure to obtain off-white solid N-butyl Sulfonyl-O-[4-(4-pyridyl)-butyl]-L-tyrosine. The weight is 53.63g, the molar yield is 74.34%, and no isomer is detected.
[0110] Isomer detectio...
Embodiment 3
[0111] The preparation of embodiment 3 tirofiban hydrochloride intermediate N-butanesulfonyl-O-[4-(4-pyridyl)-butyl]-L-tyrosine
[0112] A In dimethylsulfoxide (450ml), add 50g (0.166mol) of N-(butanesulfonyl)-L-tyrosine, 46.1g (0.224mol) of 4-(4-pyridyl) butyl chloride hydrochloride , 1.35eq), catalyst sodium iodide 2.3g, sodium hydroxide solution (0.697mol, 4.2eq) was added dropwise under stirring, after the addition was completed, the temperature was raised to 65-75°C, and the temperature was controlled at 65-75°C for 14h.
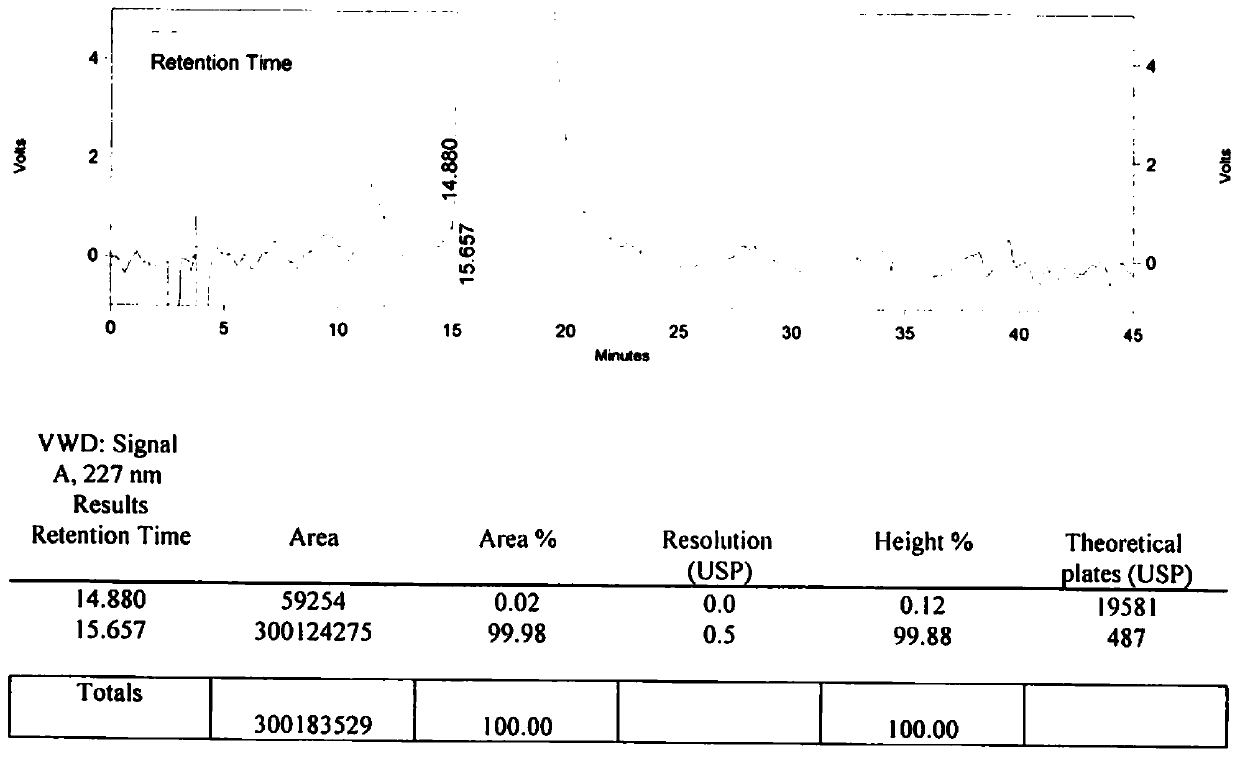
[0113] B After the reaction is completed, add 2.8L of purified water and 2.0L of dichloromethane, adjust the pH value to 5.9 with dilute hydrochloric acid, stand still and separate the phases, take the organic phase (dichloromethane phase), and concentrate under reduced pressure to obtain off-white solid N-butyl Sulfonyl-O-[4-(4-pyridyl)-butyl]-L-tyrosine. The weight is 52.54g, the molar yield is 72.84%, and the isomer content is 0.02%. See the liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com