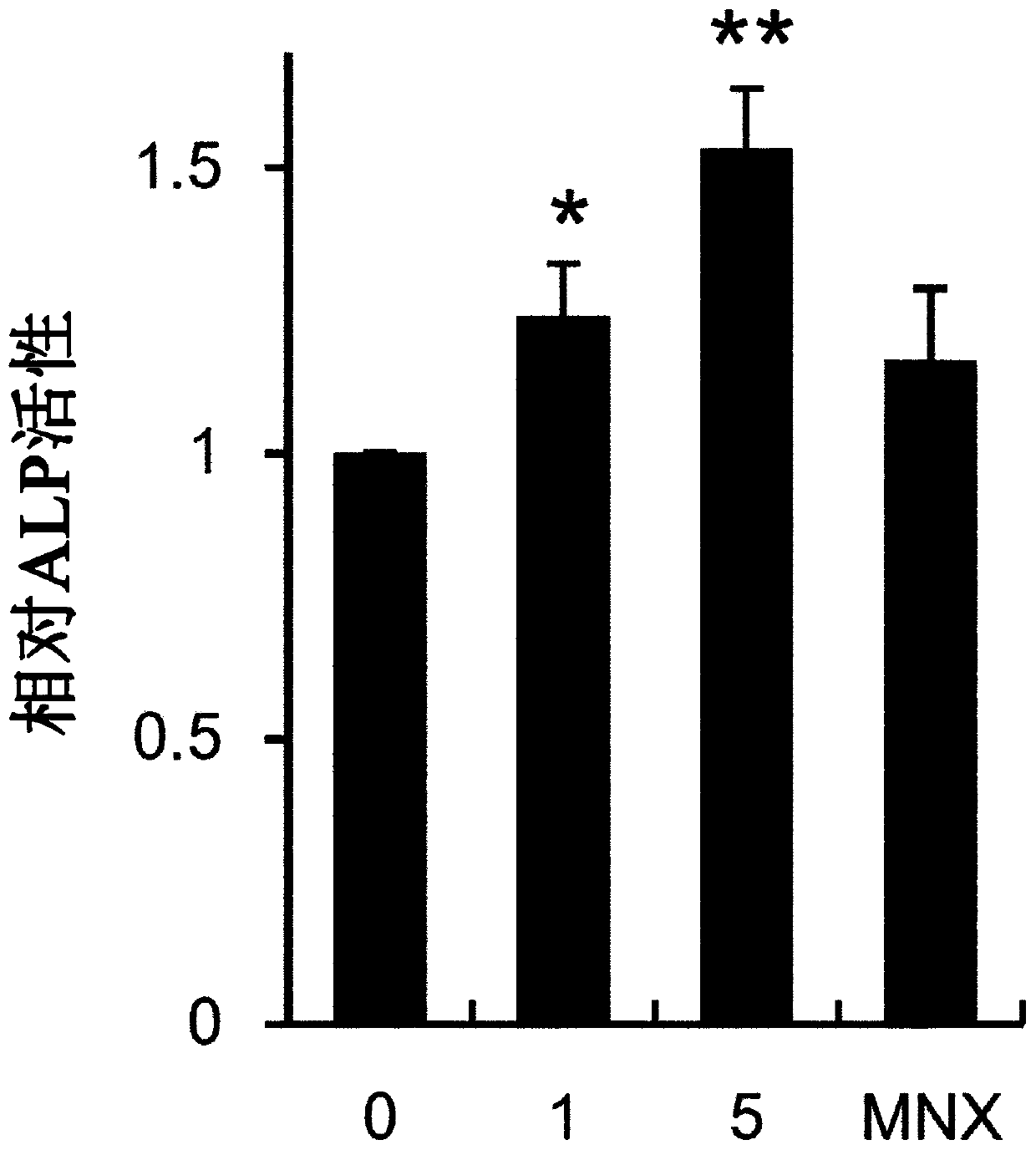
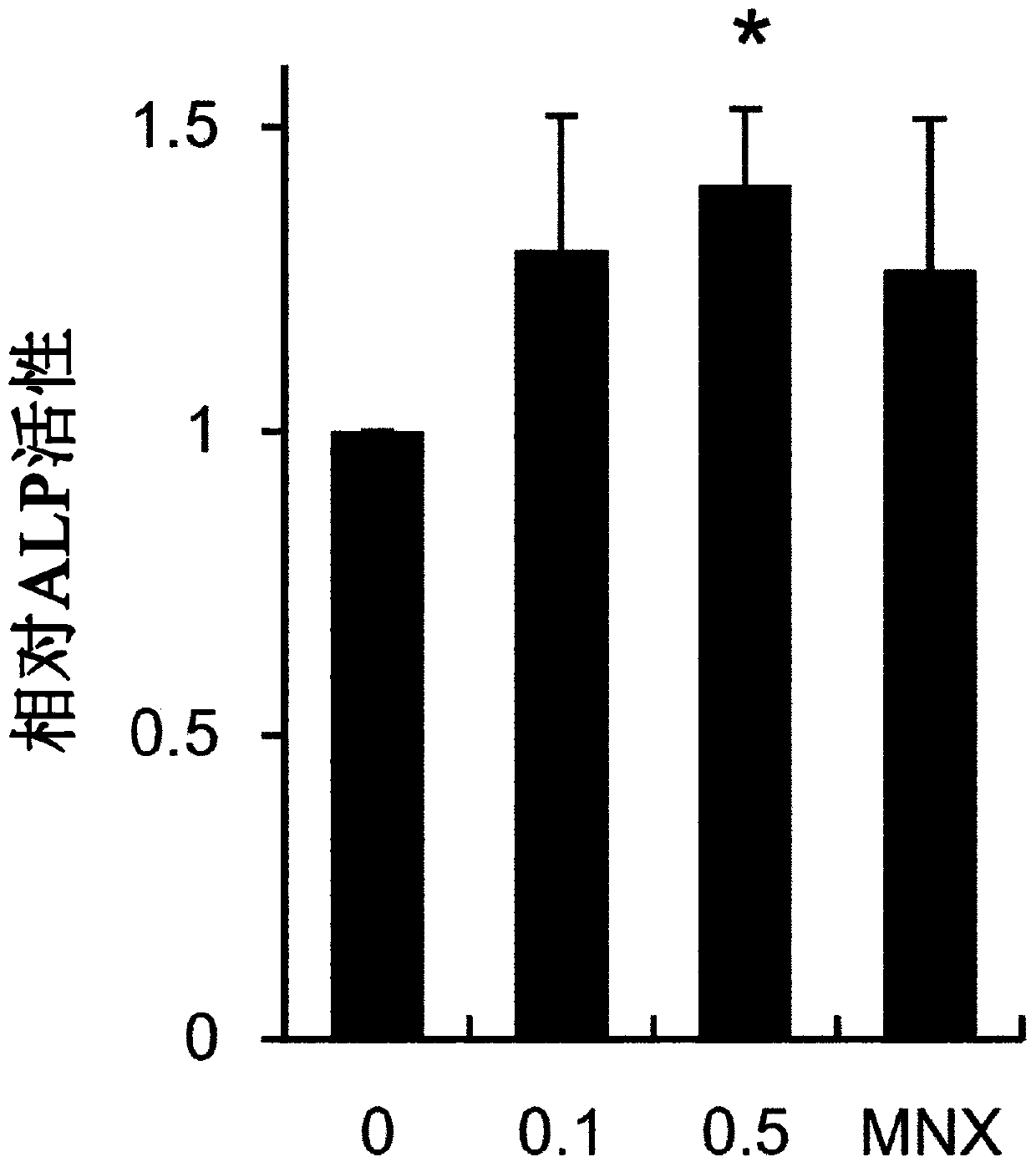
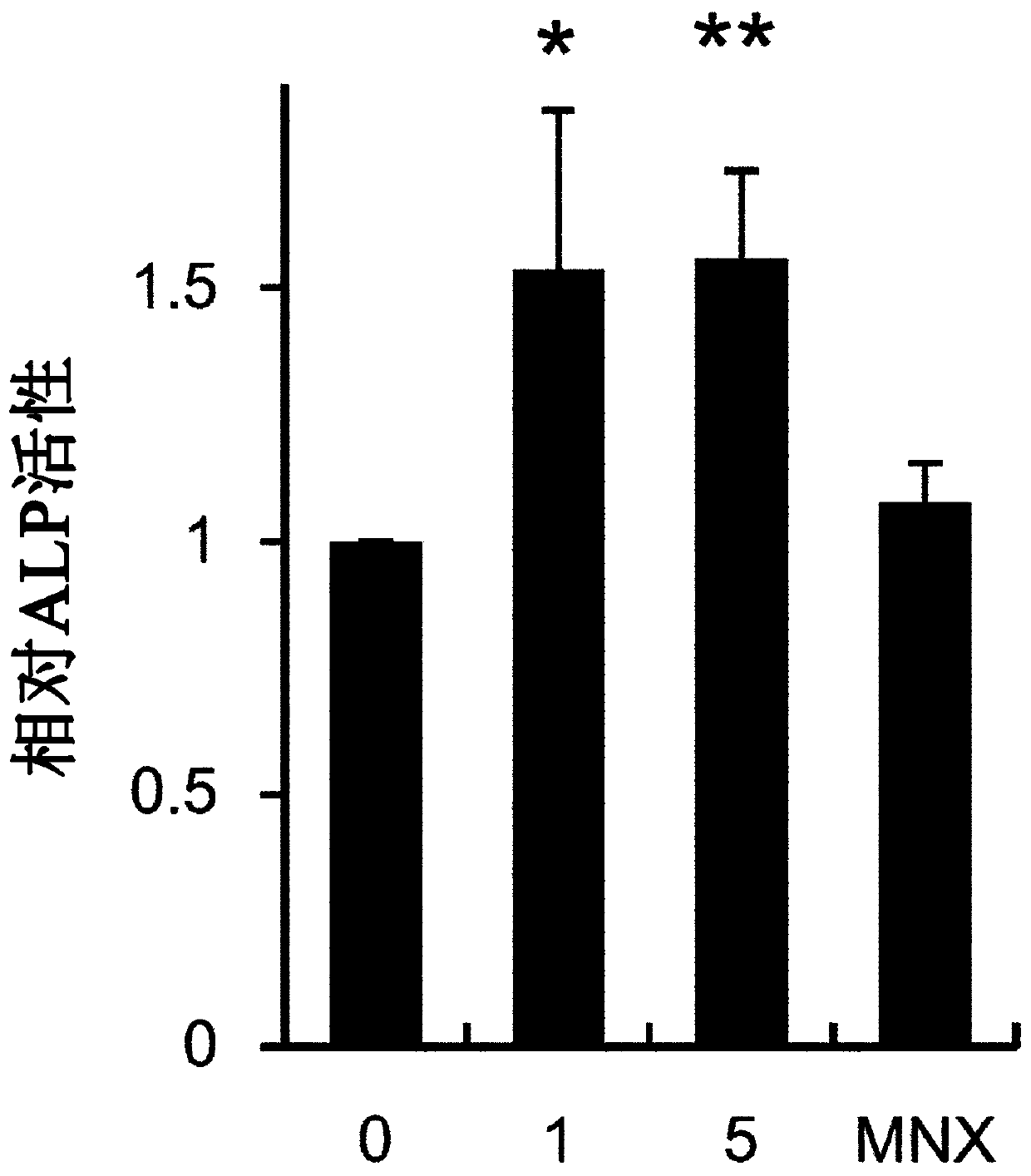
Composition for preventing hair loss or promoting hair growth
A hair growth and hair loss technology, applied in the direction of drug combination, hair care, non-active ingredients of polymer compounds, etc., can solve the problems of toxicity, side effects, and insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0187] Preparation Example 1. Synthesis of 5-methoxy indirubin-3'-oxime (A3334)
[0188] ①Synthesis of intermediate product 5'-methoxy-[2,3'-biindolineylidene]-2',3-dione
[0189]
[0190] 5-Methoxyisatin (1000mg, 5.65mmol) was added to a 250ml round bottom flask and dissolved in methanol (225ml), then indole acetate (989mg, 5.65mmol) and sodium carbonate (Na 2 CO 3 ) (1496 mg, 14.11 mmol), and the mixture was stirred at 65° C. for 12 hours. The reaction was quenched by TLC (Rf = 0.4, ethyl acetate / hexane = 1 / 2 (vol / vol)), and the product was cooled on ice until massive crystals formed. After crystals were formed and the solvent was removed by filtration, the filtrate was discarded, and the product was washed several times with solvent (ethanol / water=1 / 1 (vol / vol)). The product was filtered, dried in a vacuum pump and used in the next step without further purification.
[0191] ②Synthesis of A3334
[0192]
[0193] 5'-Methoxy-[2,3'-biindolineylidene]-2',3-dione (6...
preparation Embodiment 2
[0194] Preparation Example 2. Synthesis of 5,6-dichloroindirubin-3'-methoxime (A3051)
[0195] ①Synthesis of intermediate product 5',6'-dichloro-[2,3'-biindolineylidene]-2',3-dione
[0196]
[0197] 5,6-Dichloroisatin (500 mg, 2.32 mmol) was added to a 250 ml round bottom flask and dissolved in methanol (MeOH) (92.80 ml), followed by addition of indole acetate (405.48 mg, 2.315 mmol) and Sodium carbonate (Na 2 CO 3 ) (637.83mg, 6.02mmol), and the mixture was stirred at 65°C for 12 hours. The reaction was quenched by TLC (Rf = 0.4, ethyl acetate / hexane = 1 / 2 (vol / vol)), and the product was cooled on ice until massive crystals formed. After crystals formed, the solvent was removed by filtration. The filtrate was discarded, and the product was washed several times with solvent (ethanol / water=1 / 1 (vol / vol)). The product was filtered, dried in a vacuum pump and used in the next step without further purification.
[0198] ② Synthesis of A3051
[0199]
[0200] Charge ...
preparation Embodiment 3 and 4
[0201] Preparation Examples 3 and 4. Preparation of Indirubin Derivatives, Formula 3 (A3486) and Formula 4 (A3538).
[0202] By the same method, 5,6-dichloroindirubin-3'-methoxime or 5-methoxyindirubin-3'-oxime (dissolved in dimethyl sulfide (DMSO) and used in the experiment ) to synthesize 5,6-dichloroindirubin-3'-oxime propioxime of formula 3 and 6-chloroindirubin-3'-benzyl oxime of formula 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com