PD-L1 antibody secretion anti-mesothelin CAR-T cell tumor immunotherapy
A PD-L1, lymphocyte technology, applied in the field of biomedicine, can solve problems such as breathing difficulties of patients, and achieve the effects of enhanced proliferation and survival ability, high safety, and enhanced killing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Cell lines and basic experimental techniques used in the embodiments of the present invention are as follows:
[0108] Generation of lentivirus and transduction of human T lymphocytes
[0109] Replication-defective lentiviral vectors were generated and collected by centrifugation for transduction of human T lymphocytes. The following is a brief introduction to the production and collection of lentiviral vectors: 293T cells were placed on a cell culture dish with a bottom area of 150-cm2, and according to the instructions, Express-In (purchased from Open Biosystems / ThermoScientific, Waltham, MA) Viral transduction of 293T cells. Add 15 μg of lentiviral transgenic plasmid, 5 μg of pVSV-G (VSV glycoprotein expression plasmid), 10 μg of pCMVR8.74 plasmid (Gag / Pol / Tat / Rev expression plasmid) and 174 μl of Express -In (at a concentration of 1 μg / μl). The supernatants were collected at 24 hours and 48 hours, and centrifuged for 2 hours using an ultracentrifuge at 28,000 r...
Embodiment 2
[0114] Example 2 Construction of vectors that co-express anti-PD-L1 single-chain antibody and IgG Fc fusion protein containing amino acid mutations and anti-MSLN chimeric antigen receptor
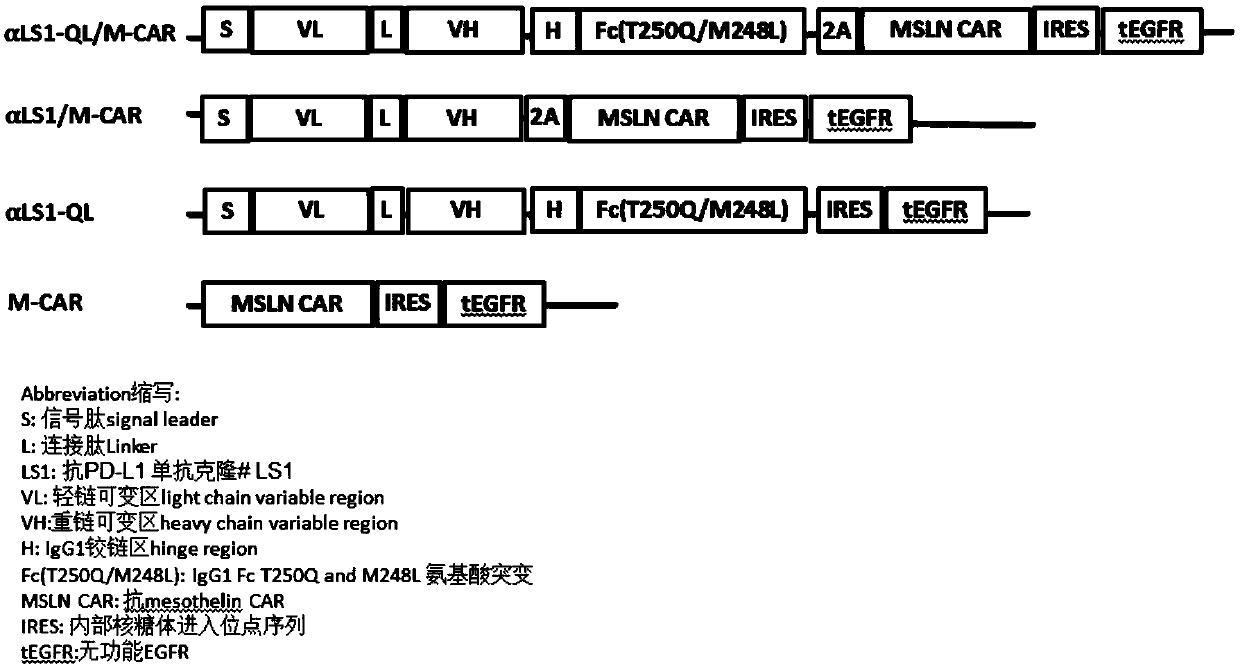
[0115] In this example, the inventors cloned the sequence encoding the single-chain antibody against human MSLN, the ζ-chain sequence of the 4-1BB intracellular segment and the T cell receptor combination into a lentiviral vector containing the EF-1 promoter ( lentiviral vector), during the cloning process, the selected restriction enzymes were XbaI and NotI double enzyme digestion, and NotI and XhoI double enzyme digestion. Lentiviral plasmid with antigen receptor complex (LV-MSLN CAR). The sequences of the anti-PD-L1 single-chain antibody (LS1clone) and the IgG Fc fusion protein containing amino acid mutations were cloned into the LV-MSLNCAR vector plasmid to construct a lentiviral vector αLS1-QL / M-CAR. figure 1 It is a schematic diagram of the lentiviral vector, including the sequence e...
Embodiment 3
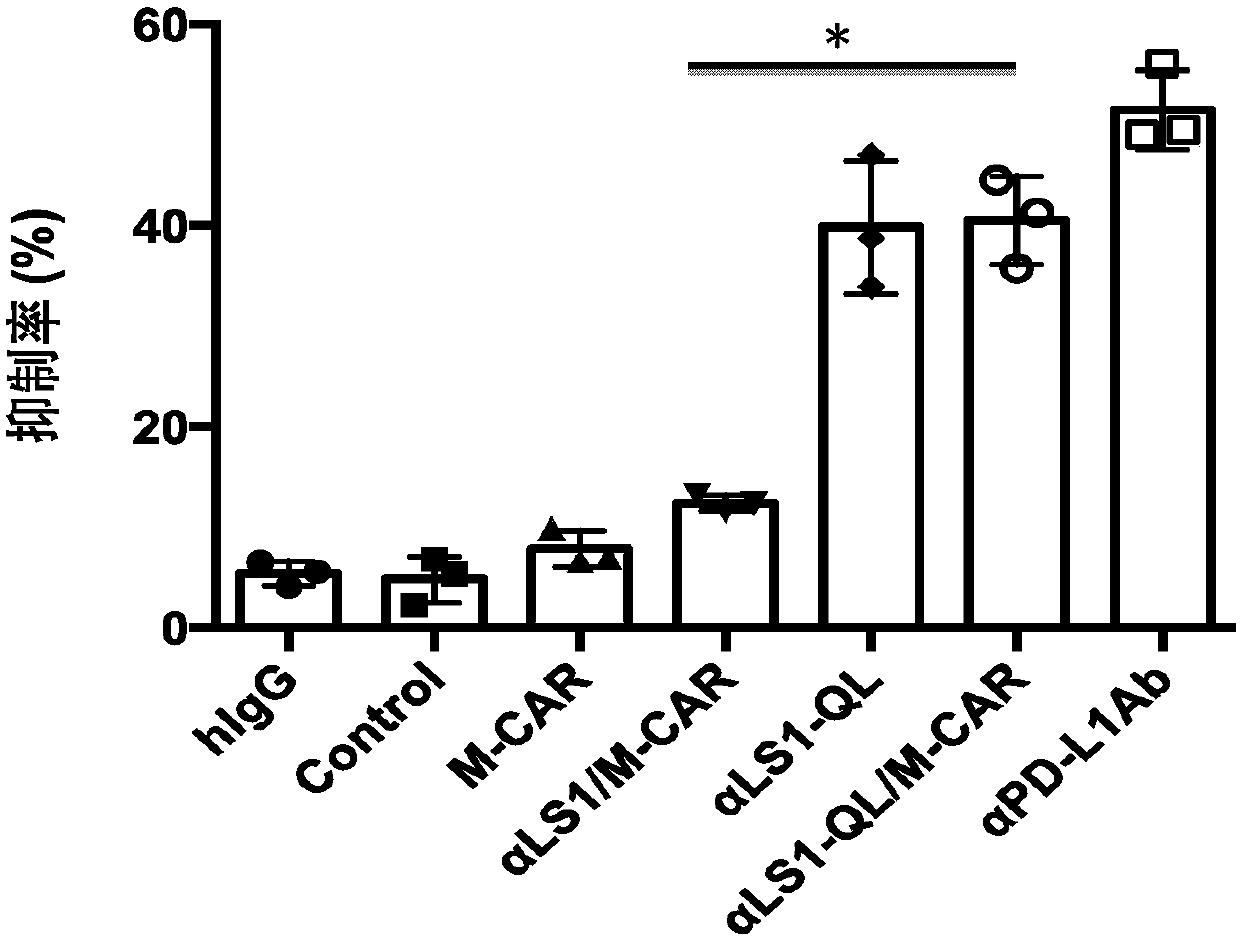
[0116] Example 3 Vector αLS1-QL / M-CAR transduces T lymphocytes to secrete anti-PD-L1 single-chain antibody and IgG Fc fusion protein containing amino acid mutations to inhibit the effect of PD-1:PD-L1
[0117] In this example, peripheral blood lymphocytes were obtained from anonymous blood donors. Peripheral blood lymphocytes were separated by gradient centrifugation using Ficoll-Hypaque. In the presence of T lymphocyte activating factor magnetic beads CD3 / CD28 (purchased from Invitrogen, Carlsbad, CA), activated T lymphocytes were transduced with lentiviral vectors, expanded and cultured in vitro, and the method was as described in Example 1. 3-7 days after lentiviral vector transduction, harvest the transduced T cells (the number of cells is 2×10 6 / well) culture supernatant for PD-1:PD-L1 inhibition experiment. 100 ng / well of PD-L1 protein (Aerobiosystems, Boston, MA) was used to coat 96-well ELISA plates. 10ng of biotin-labeled PD1 (Aerobiosystems, Boston, MA) was mixed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com