Recombinant humanized III-type collagen and prokaryotic expression method thereof
A collagen and expression carrier technology, applied in the field of recombinant human type III collagen and its prokaryotic expression, can solve the problems of not being able to copy human collagen well, achieve good biological activity and promote cell migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 gene design and synthesis
[0031] (1) Gene design: According to the amino acid sequence of human type III collagen (UniProtKB / Swiss-Prot:P02461.4), SEQ ID NO: 1:
[0032] MYDSYDVKSGVAVGGLAGYPGPPGPPPGPAGPPGPPGPPGTSGHPGSPGSPGYQGPPGEPGQAGPSGPPGPPGAIGPSGPAGKDGESPGGRPGRPGERGLPGPPGIKGPAGIPGFPGMKGHRGFDGRNGEKGETGAPGLKGENGLPGENGAPGPMGPRGAPGERGRPGLPGAAGARGNDGARGSDGQPGPPGPPGTAGFPGSPGAKGEVGPAGSPGSNGAPGQRGEPGPQGHAGPPGPVGPAGKSGDRGESGPAGPAGAPGPAGSRGAPGPQGPRGDKGETGERGAAGIKGHRGFPGNPGAPGSPGPAGQQGAIGSPGPAGPRGPVGPSGPPGKDGTSGHPGPIGPPGPRGNRGERGSEGSPGHPGQPGPPGPPGAPGPCCGGVGAAAIAGIGGEKAGGFAPYYHHHHHH
[0033] Use the online design tool Jcat (http: / / www.jcat.de / ) to reverse design the gene sequence, aiming at the preferred codons required for expression in the host Escherichia coli, and remove the NdeI and XhoI restriction sites during the design process, which is beneficial to the later stage Gene manipulation, the optimized gene sequence is shown as SEQ ID NO: 2, compared with the...
Embodiment 2
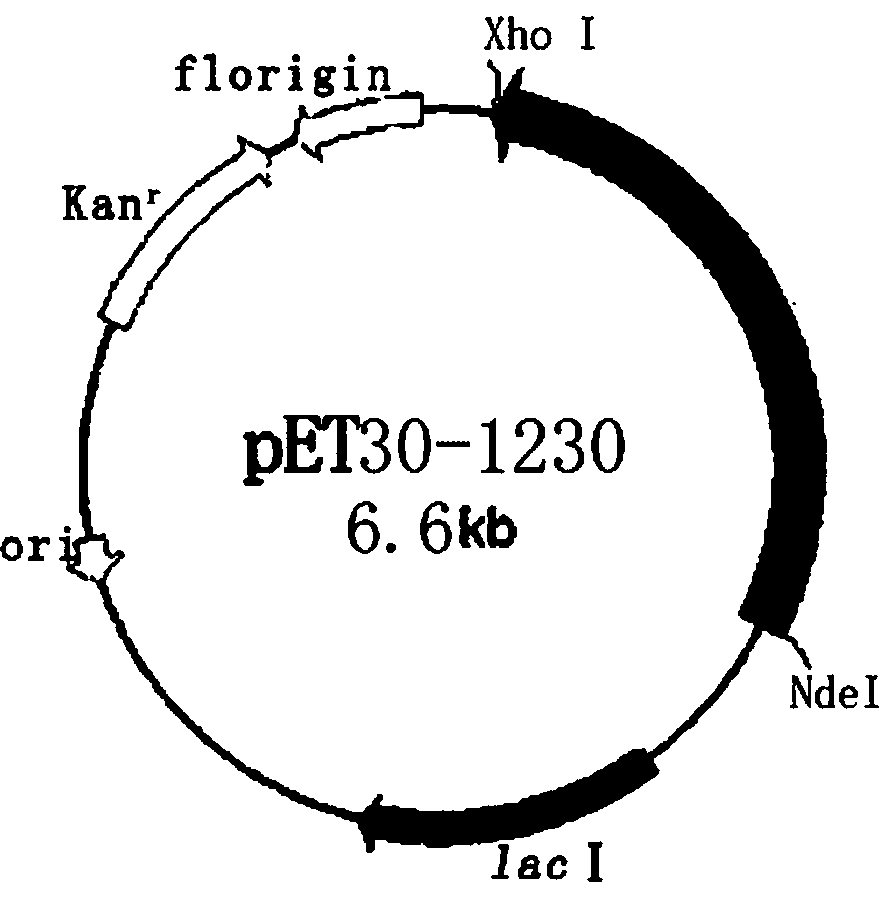
[0038] Example 2 Construction of recombinant expression vector pET30-1230
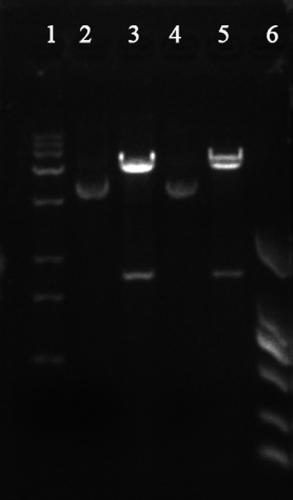
[0039] Use NdeI and XhoI double enzymes to digest the gene SEQ ID NO:2 fragment obtained in Example 1. The enzyme digestion system is as follows: 50 μL of enzyme digestion system, 5 μL (2 μg) of SEQ ID NO:2 fragment, 1 μL each of NdeI and XhoI enzymes, buffer 5 μL solution, supplemented with 50 μL sterilized double distilled water, and incubated at 37°C for 4 hours. Use the same system to digest plasmid PET30a (+). The linear fragment was obtained by enzyme digestion, the target fragment was purified by DNA gel recovery kit, and the obtained fragment was ligated with T4 ligase. The ligation system was as follows: T4 DNA ligase 1 μL, 1230 digested fragment 3 μL, PET30a (+) 2 μL of digested fragments, 1 μL of ligation buffer, and 10 μL of sterilized double-distilled water. Incubate at 16°C for 4h. Transform the incubated product into host bacteria by heat shock method E. coli In DH5α, apply to LB cu...
Embodiment 3
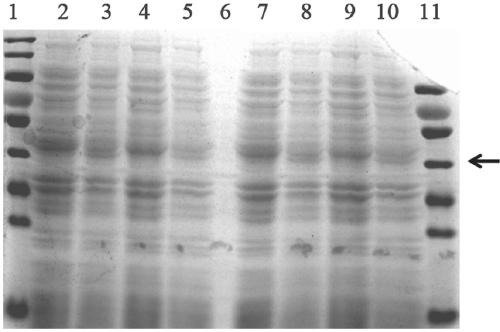
[0040] Example 3 Construction of Engineering Bacteria
[0041] Pick a single colony of Escherichia coli BL21 (DE3) and inoculate it in an LB test tube, then shake overnight at 37°C; add 0.5mL overnight culture solution to a Erlenmeyer flask containing 50mL LB, and shake vigorously at 37°C for about 2 hours to grow the bacteria to In the early logarithmic period; under sterile conditions, transfer the bacteria to a 50mL polypropylene tube pre-cooled with ice, and place it on ice for 10 minutes; centrifuge at 4°C, 4000rpm, pour off the supernatant, and invert the tube to make the residual liquid flow out as much as possible; add 6mL 0.1mol / L CaCl pre-cooled with ice 2 Resuspend the pellet and place it on ice for 30min; centrifuge at 3000rpm at 4°C, pour off the supernatant, invert the tube to make the residual liquid flow out as much as possible; add 1.2mL of 0.1mol / L CaCl pre-cooled with ice 2 Resuspend the pellet (if you want to prepare competent cells for storage at -70°C, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com