Polydopamine multi-level nano-drug carrier, and preparation method and application thereof
A polydopamine multi-level, nano-drug carrier technology, applied in the field of nano-materials, can solve the problems of poor stability, poor magnetic response, and low coating degree of nanoparticles, and achieve good photostability, high coating degree, and magnetic response. good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
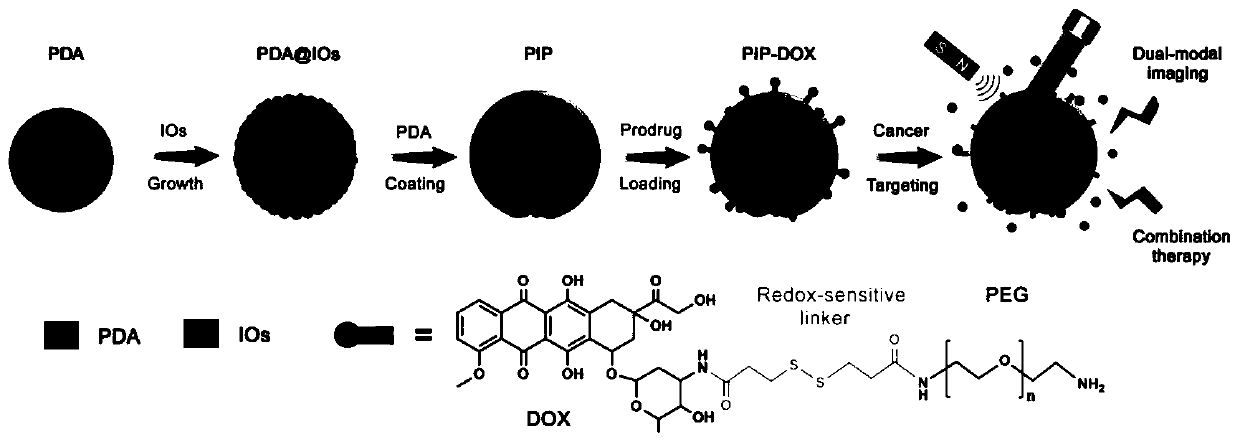
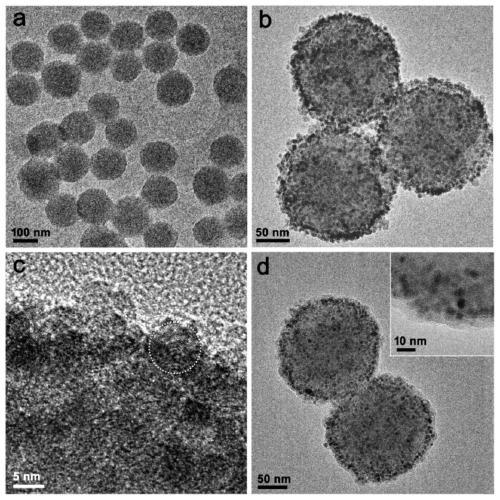
[0035] (1) Measure 40mL of ethanol, 90mL of ultrapure water and 2.2mL of ammonia water, and pour them into a 250mL reaction bottle. Weigh 0.5 g of dopamine hydrochloride and dissolve it in 10 mL of water, quickly add it into the above mixed solution under vigorous stirring, and magnetically stir at room temperature for 30 h. The solution was centrifuged to collect the precipitate and washed several times with water to obtain PDA microspheres.
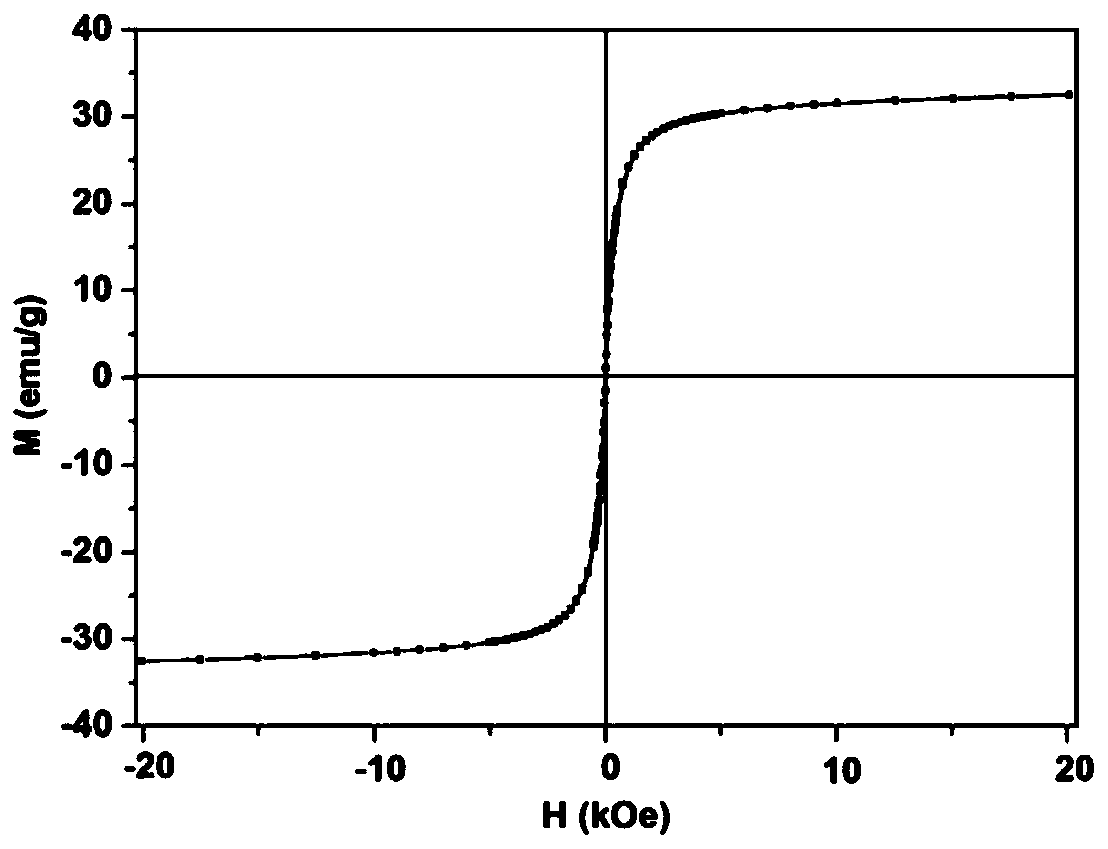
[0036] (2) The PDA microspheres prepared above (about 40 mg in dry weight) were dissolved in 1 mL of ethanol and added to a 50 mL two-necked reaction flask. Then add 240mg Fe(acac) 3 and 20 mL of TEG. The temperature of the solution was raised to 70 °C under vacuum and maintained for more than 15 min to remove ethanol. Fill the bottle with nitrogen and raise the temperature to 210°C and magnetically stir for 2h; continue to rise to 285°C and stir for 1h. After the solution was cooled to room temperature, an equal volume of acetone w...
Embodiment 2
[0042] (1) Measure 40mL of ethanol, 90mL of ultrapure water and 2.2mL of ammonia water, and pour them into a 250mL reaction bottle. Weigh 0.5 g of dopamine hydrochloride and dissolve it in 10 mL of water, quickly add it into the above mixed solution under vigorous stirring, and magnetically stir at room temperature for 30 h. The solution was centrifuged to collect the precipitate and washed several times with water to obtain PDA microspheres.
[0043] (2) The above-prepared wet precipitate (about 40 mg in dry weight) was dissolved in 1 mL of ethanol and added to a 50 mL two-necked reaction flask. Then 120 mg Fe(acac)3 and 20 mL TEG were added. The temperature of the solution was raised to 70 °C under vacuum and maintained for more than 15 min to remove ethanol. Fill the bottle with nitrogen and raise the temperature to 210°C and magnetically stir for 2h; continue to rise to 285°C and stir for 1h. After the solution was cooled to room temperature, an equal volume of acetone ...
Embodiment 3
[0048] (1) Measure 40mL of ethanol, 90mL of ultrapure water and 0.7mL of ammonia water, and pour them into a 250mL reaction bottle. Weigh 0.5 g of dopamine hydrochloride and dissolve it in 10 mL of water, quickly add it into the above mixed solution under vigorous stirring, and magnetically stir at room temperature for 30 h. The solution was centrifuged to collect the precipitate and washed several times with water to obtain PDA microspheres.
[0049] (2) The above-prepared wet precipitate (about 40 mg in dry weight) was dissolved in 1 mL of ethanol and added to a 50 mL two-necked reaction flask. Then add 240mg Fe(acac) 3 and 20 mL of TEG. The temperature of the solution was raised to 70 °C under vacuum and maintained for more than 15 min to remove ethanol. Fill the bottle with nitrogen and raise the temperature to 210°C and magnetically stir for 2h; continue to rise to 285°C and stir for 1h. After the solution was cooled to room temperature, an equal volume of acetone was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com