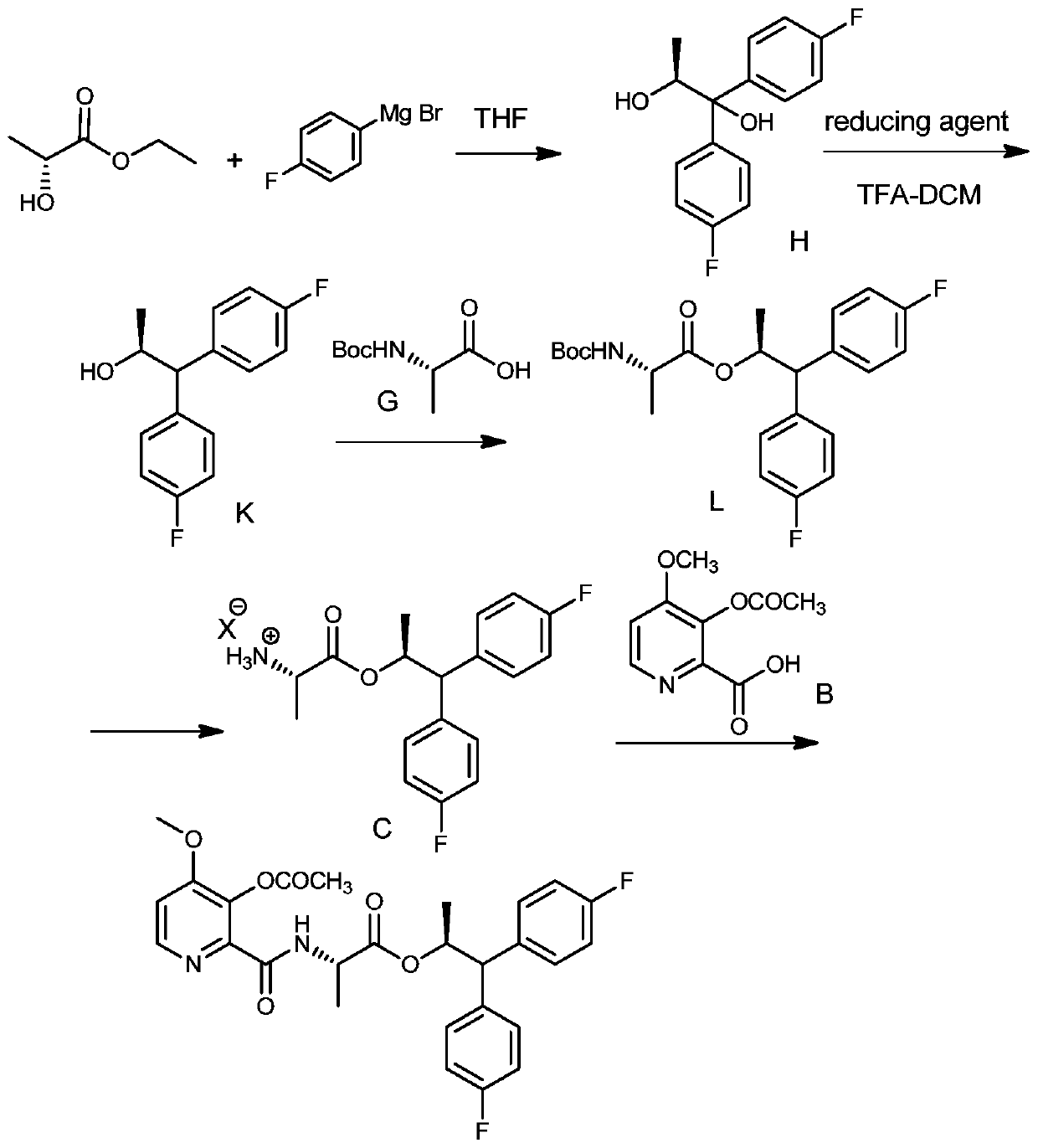
Application of ketoreductase in preparation of (S)-1,1-bis(4-fluorophenyl)-2-propanol, and preparation method of (S)-1,1-bis(4-fluorophenyl)-2-propanol
A technology of reductase and fluorophenyl, which is applied to the use and preparation field of ketoreductase in the preparation of -1,1-di-2-propanol, can solve the problems of high raw material cost, environmental pollution, etc., and achieve raw material cost reduction, high stereoselectivity, and environmentally friendly production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
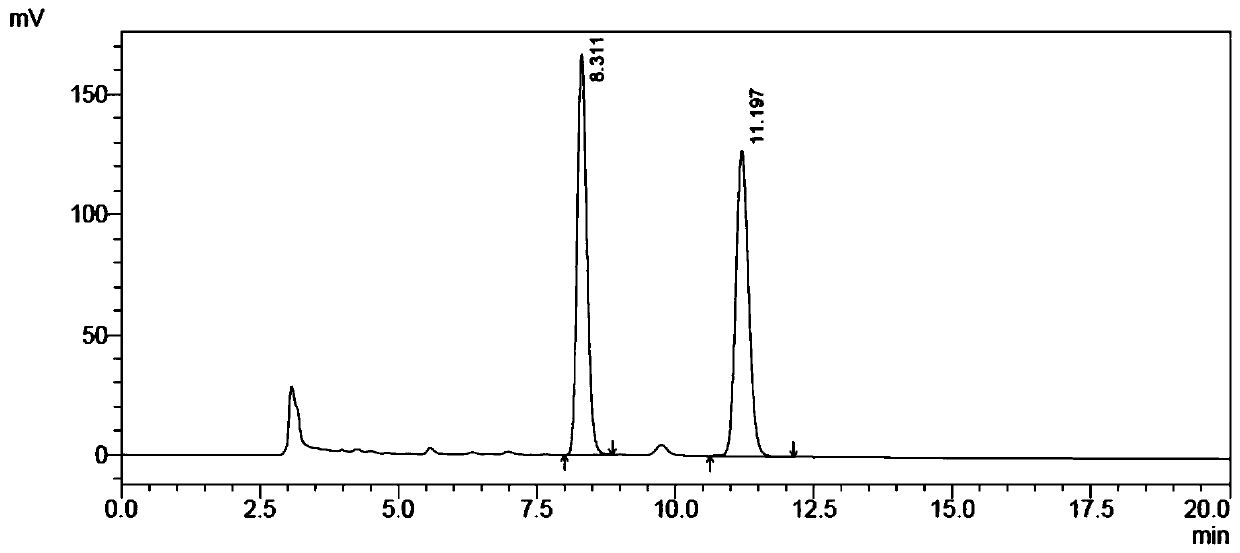
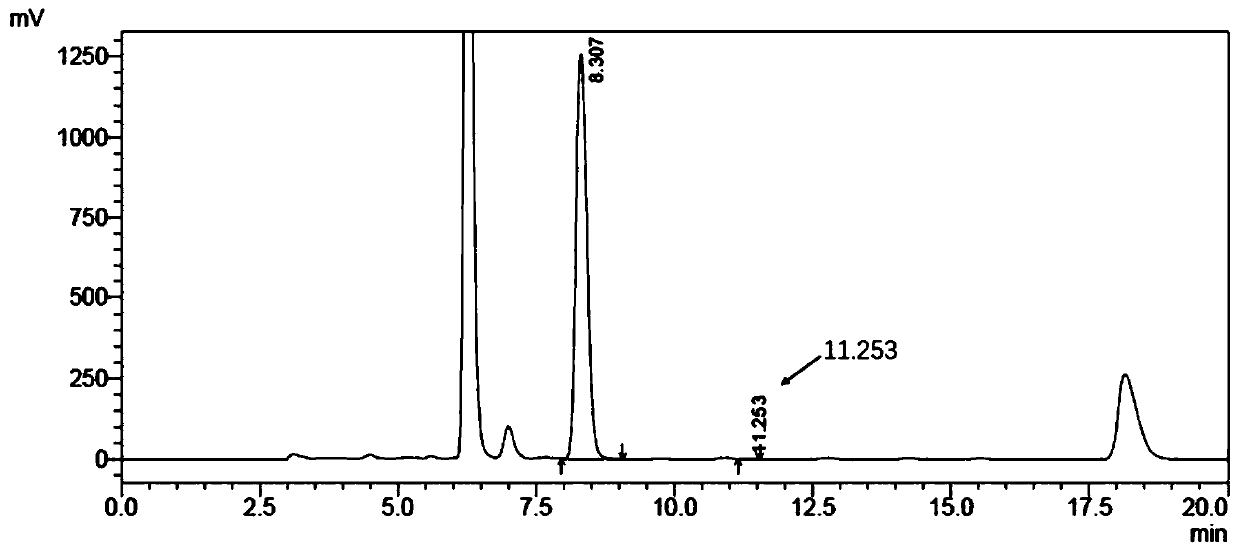
Image
Examples
Embodiment 1
[0056] Synthesis of embodiment 1 substrate formula 2 compound
[0057]
[0058] At 0-5°C, 55.6 mL (3.5 eq) of 0.8 mol / L p-fluorophenylmagnesium bromide was added dropwise to a THF (tetrahydrofuran, 15 mL) solution containing 1.5 g of ethyl lactate (1.0 eq), and the addition was completed , heated to reflux for 1.5h, then cooled to room temperature (generally 10-30°C, preferably 25°C) and stirred overnight at this temperature. TLC (thin-layer chromatography analysis) shows that the raw material disappears and the reaction is completed. The reaction solution is poured into ice water precooled 40% (mass ratio) acetic acid aqueous solution (10mL) to quench and stir for 10min, then add EA (ethyl acetate, 20mL ) was extracted twice, the organic phases were combined and dried with anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 4.6 g of crude compound of formula 3 (1,1-bis(4-fluorophenyl)-1,2-propanediol). Submit directly to the next step without purif...
Embodiment 2
[0062] The preparation of embodiment 2 KRED enzyme
[0063] 2.1 Acquisition of KRED enzyme (Ketoreductase, ketoreductase) gene
[0064] KRED gene was synthesized according to the gene sequence of ketoreductase reported on NCBI (see Table 1 for details) SEQ ID NO: 2, 4, 6, 8, 10, 12, 14.
[0065] Table 1 Amino acid sequence and nucleotide sequence of KRED enzymes from different sources
[0066]
[0067] 2.2 Preparation of KRED enzyme
[0068] The synthetic KRED gene was connected to the pET28a vector, and the enzyme-linked vector was transformed into the host E.coli BL21 (DE3) competent cells, and the engineered strain containing the KRED gene was obtained.
[0069] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use.
[0070] After the engineering bacteria containing the KRED gene were activated by streaking on a plate, a single colony was picked...
Embodiment 3
[0081] Example 3 Acquisition and expression of alcohol dehydrogenase gene
[0082] According to the Cyclopentanol dehydrogenase gene sequence (as shown in SEQ ID NO: 15) derived from Lactobacillus brevis KB290 (Genbank accession number: BAN05992.1), the alcohol dehydrogenase gene was synthesized completely.
[0083] The alcohol dehydrogenase gene is connected to the pET28a vector, and the enzyme-linked vector is transformed into the host E.coli BL21 (DE3) competent cells, and the engineering strain containing the alcohol dehydrogenase gene is obtained. After the engineering bacteria containing the alcohol dehydrogenase gene were activated by streaking on the plate, a single colony was picked and inoculated into 5ml LB liquid medium containing 50μg / ml kanamycin, and cultured with shaking at 37°C for 12h. Transfer to 50ml fresh LB liquid medium also containing 50μg / ml kanamycin according to 2% inoculum size, and shake to OD at 37°C 600 When it reaches about 0.8, add IPTG to its...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com