Alcohol dehydrogenase mutant and application thereof
An alcohol dehydrogenase and mutant technology, which is applied in the field of enzyme engineering to achieve the effects of improving catalytic activity, improving activity expression rate, and improving fermentation enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Microbial Culture and Enzyme Activity Determination
[0032] 1.1 Culture of microorganisms
[0033] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use. LB solid medium (plate petri dish): add 20g / L agar powder on the basis of LB liquid medium, sterilize at 121°C, cool down, and introduce into a petri dish to make a flat plate.
[0034] The engineered bacteria E.coli BL21(DE3) containing related genes were inoculated into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 12 hours. Transfer to 500mL fresh LB liquid medium also containing 50μg / mL Kan, shake culture at 37°C until OD 600 When it reaches about 0.8, add IPTG to its concentration of 0.3mM, and induce culture at 28°C for 20h. After the cultivation, the culture solution was centrifuged at 10,000 rpm for 10 min, the supernatant was dis...
Embodiment 2
[0041] Example 2 Construction of Escherichia coli Genetically Engineered Bacteria Overexpressing Molecular Chaperone GroES-EL
[0042] Using CRISPR gene editing technology, the weak promoter σ26-σ32 (gene sequence is SEQ ID NO.4) at the front end of the expression molecular chaperone GroES-EL gene in the genome of Escherichia coli engineering bacteria was replaced with a higher strength constitutive promoter J23104 ( Sourced from http: / / parts.igem.org / Promoters / Catalog / Anderson, the gene sequence is SEQID NO.5).
[0043] The specific operation steps are as follows:
[0044] (1) Preparation of Escherichia coli electroporation competent cells carrying pCas plasmid
[0045] 1) Preparation of competent cells for transduction: Pick a single colony of E. coli E coli BL21 (DE3) and culture it in a 5 mL LB liquid medium test tube with a kanamycin concentration of 50 μg / mL for 6-8 hours, then use 2% inoculum Inoculate in a 50mL Erlenmeyer flask, incubate at 37°C, 200rpm for 2h, and p...
Embodiment 3
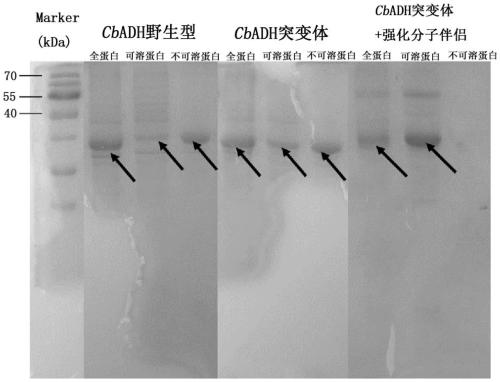
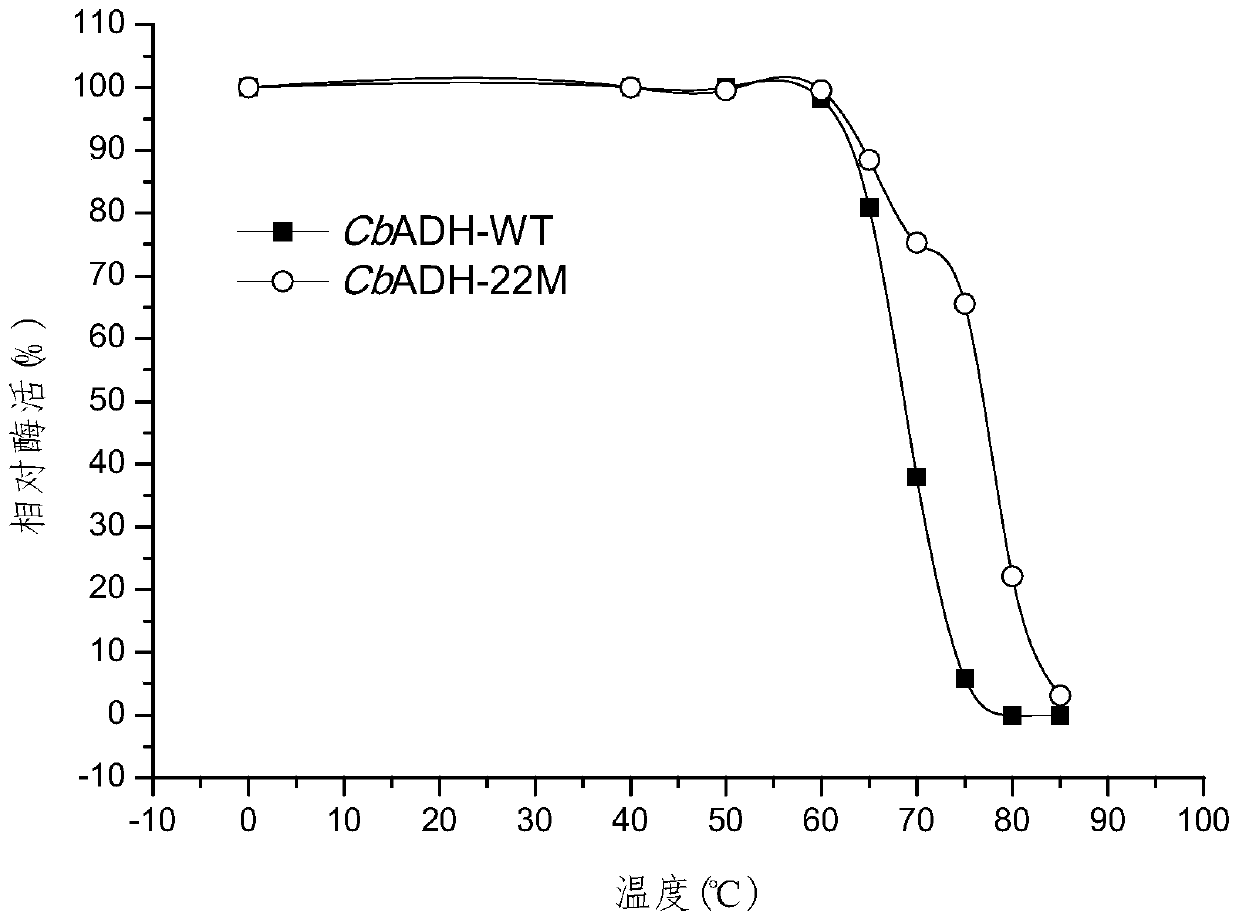
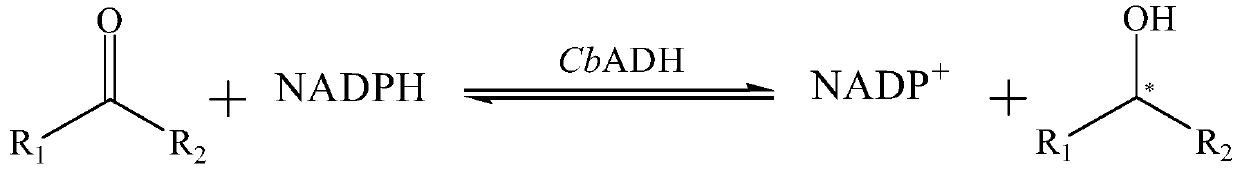
[0097] Example 3 Construction of CbADH wild-type and mutant recombinant bacteria
[0098] Entrusted Beijing Qingke Xinye Biotechnology Co., Ltd. to provide codon optimization and gene synthesis services, and synthesized CbADH wild-type (NCBI accession number WP_077844196.1) and mutant genes (SEQ ID NO.3) in pET-28a (+ ) plasmid with the sequence for coding 6×His tag (located at the C-terminus of the protein), which is convenient for protein purification, and the target gene is placed between the enzyme cutting sites Nco I and Xho I. Obtain pET-28a (+)-CbADH-WT and pET-28a (+)-CbADH-22M recombinant plasmid, and it is respectively transformed into the Escherichia coli genetic engineering bacterium (enhanced expression molecular chaperone GroES-EL) obtained in embodiment 2 )middle.
[0099] The amino acid sequence of the wild-type CbADH protein expressed by the wild-type gene sequence is shown in SEQ ID NO.1, the amino acid sequence of the mutant CbADH protein expressed by the m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pre-denatured | aaaaa | aaaaa |
| Extend | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com