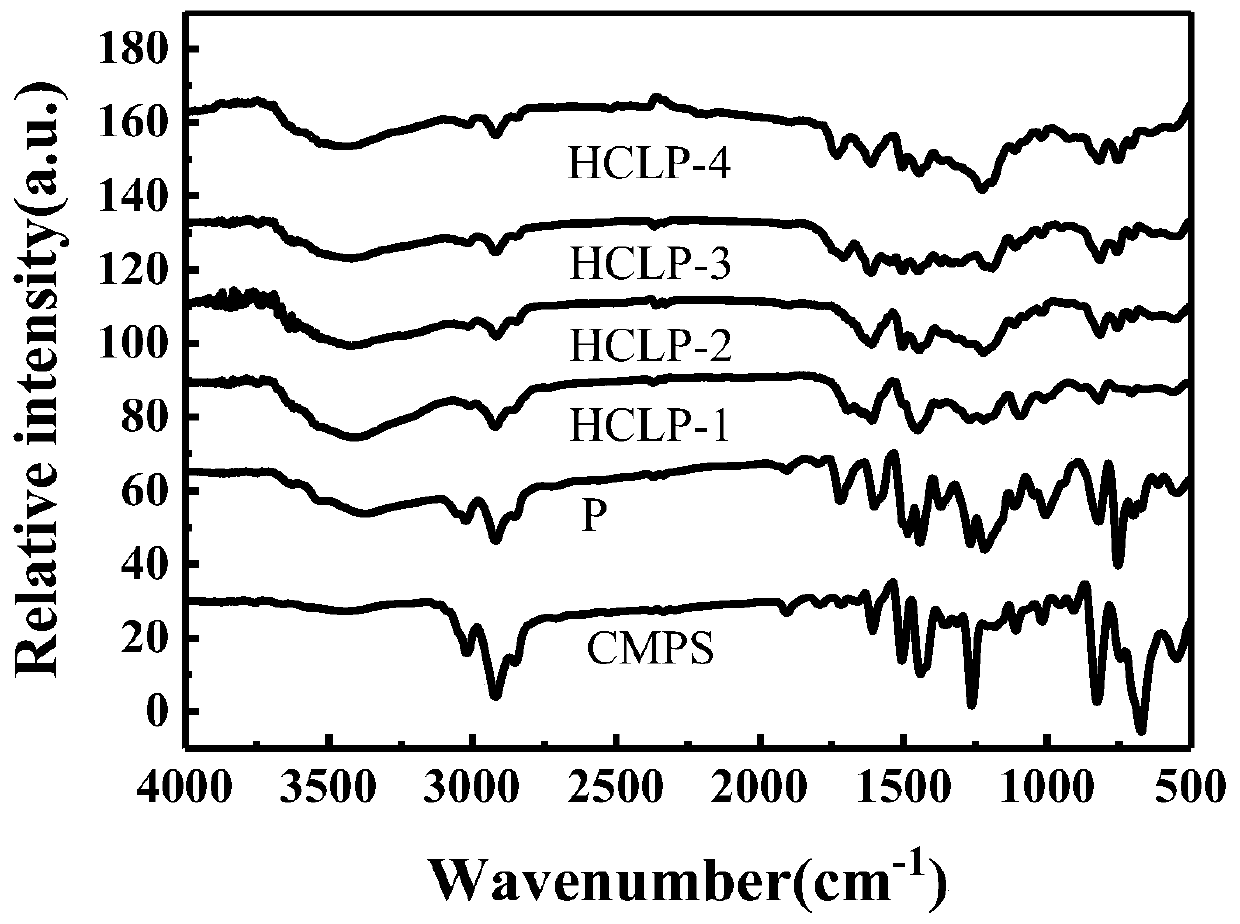
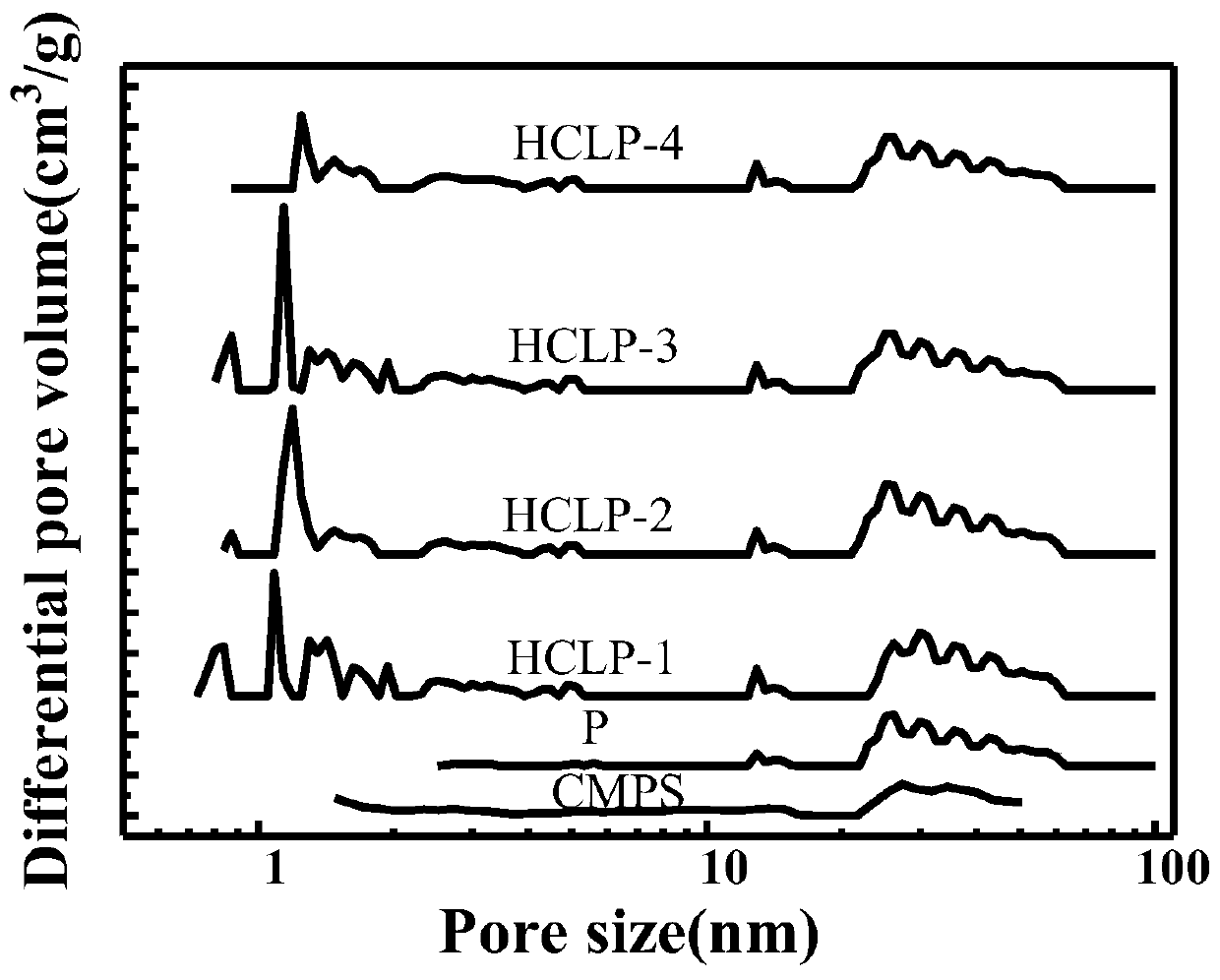
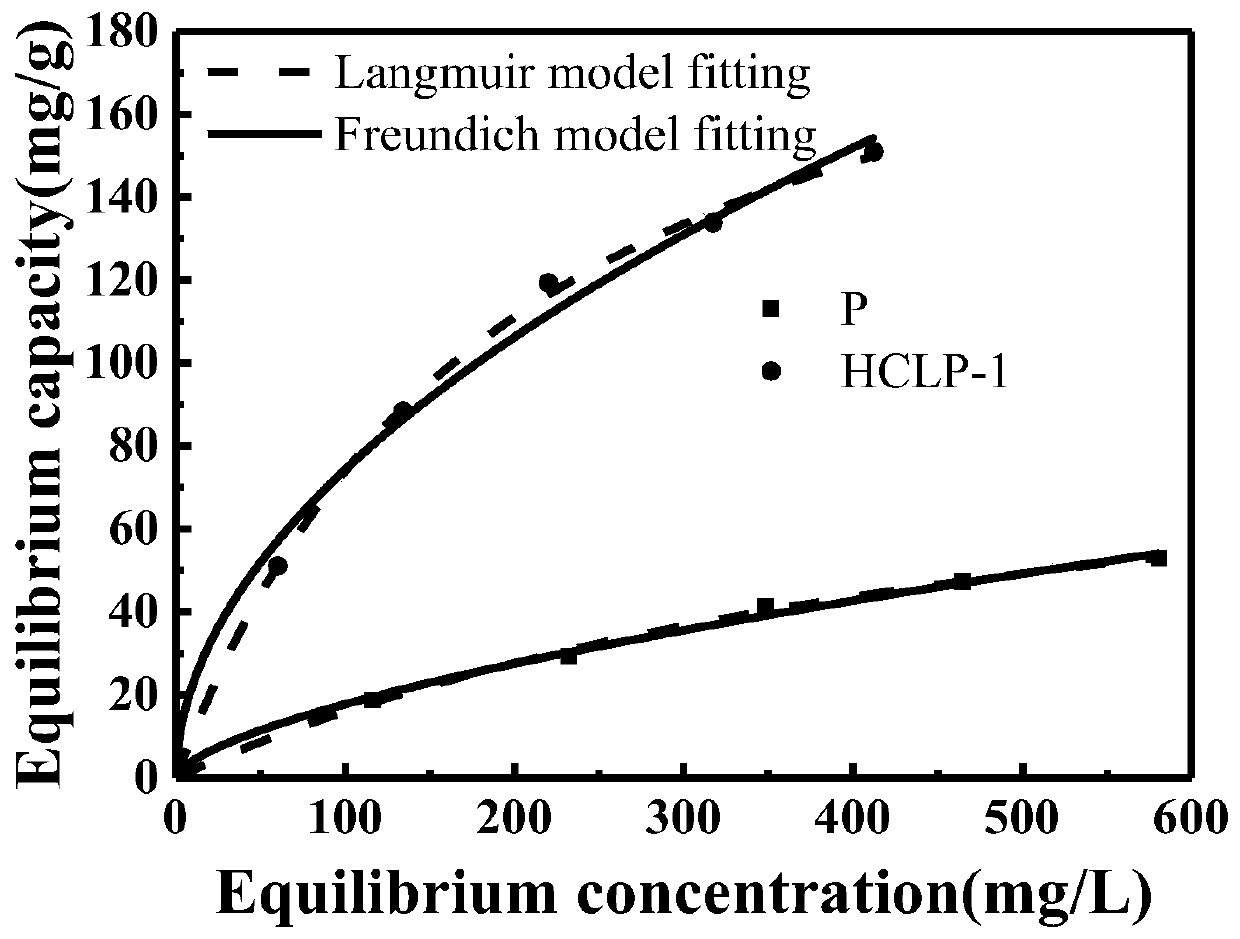
Preparation method of hydrogen bond acceptor oxygen atom-rich super-crosslinked polymer and application of super-crosslinked polymer
A polymer and super-crosslinking technology, applied in the direction of alkali metal oxides/hydroxides, separation methods, alkali metal compounds, etc., can solve the problem of difficult to obtain super-crosslinked polymers, limited improvement ability, limited modification ability, etc. problems, to achieve the effect of improving selective adsorption capacity, increasing selective adsorption capacity, and improving selective adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) Preparation of precursor polymers rich in hydrogen bond acceptor oxygen atoms:
[0039] Add 20 g of chloromethylated polystyrene low-crosslinked polymer to N,N-dimethylformamide (DMF), and seal and swell overnight at room temperature. Install a reflux condenser and a mechanical stirrer, add phenol and 10g of basic catalyst K at room temperature 2 CO 3 , stirred for 30 minutes until completely dissolved, raised the temperature to 90-95°C, and reacted at this temperature for 24 hours, washed the obtained product alternately with hot water, cold water, and absolute ethanol for 3-4 times until colorless, and then washed with ethanol, The volume ratio of methanol and water is 1:1:1, extracting in a Soxhlet extractor for 8-24 hours, ordinary drying for 12 hours, and then vacuum drying for 24 hours to obtain the precursor polymer P;
[0040] 2) Friedel-Crafts alkylation reaction:
[0041] Add 10 g of precursor polymer P and 120 mL of 1,2-dichloroethane into a dry three-n...
Embodiment 2
[0052] 1) Preparation of precursor polymers rich in hydrogen bond acceptor oxygen atoms:
[0053] Add 20 g of chloromethylated polystyrene low-crosslinked polymer to N,N-dimethylformamide (DMF), and seal and swell overnight at room temperature. Install reflux condenser and mechanical stirrer, add naphthol and 10g basic catalyst K at normal temperature 2 CO 3 , stirred for 30 minutes until completely dissolved, raised the temperature to 90-95°C, and reacted at this temperature for 24 hours, washed the obtained product alternately with hot water, cold water, and absolute ethanol for 3-4 times until colorless, and then washed with ethanol, The volume ratio of methanol and water was 1:1:1, extracted in a Soxhlet extractor for 8-24 hours, dried for 12 hours in a normal way, and then dried in vacuum for 24 hours to obtain the precursor polymer P; infrared characterization showed that the precursor polymer P was -1 The absorption peak corresponding to the C-Cl stretching vibration ...
Embodiment 3
[0063] 1) Preparation of precursor polymers rich in hydrogen bond acceptor oxygen atoms:
[0064] Add 20 g of chloromethylated polystyrene low-crosslinked polymer to N,N-dimethylformamide (DMF), and seal and swell overnight at room temperature. Install reflux condenser and mechanical stirrer, add catechol and 10g basic catalyst Na at normal temperature 2 CO 3 , stirred for 30 minutes until completely dissolved, raised the temperature to 90-95°C, and reacted at this temperature for 24 hours, washed the obtained product alternately with hot water, cold water, and absolute ethanol for 3-4 times until colorless, and then washed with ethanol, The volume ratio of methanol and water was 1:1:1, extracted in a Soxhlet extractor for 8-24 hours, dried for 12 hours in a normal way, and then dried in vacuum for 24 hours to obtain the precursor polymer P; infrared characterization showed that the precursor polymer P was -1 The absorption peak corresponding to the C-Cl stretching vibration...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com