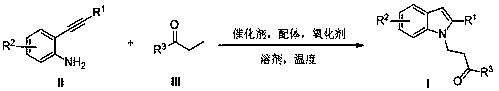
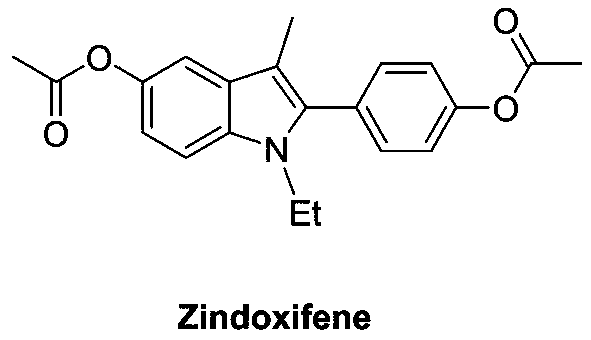
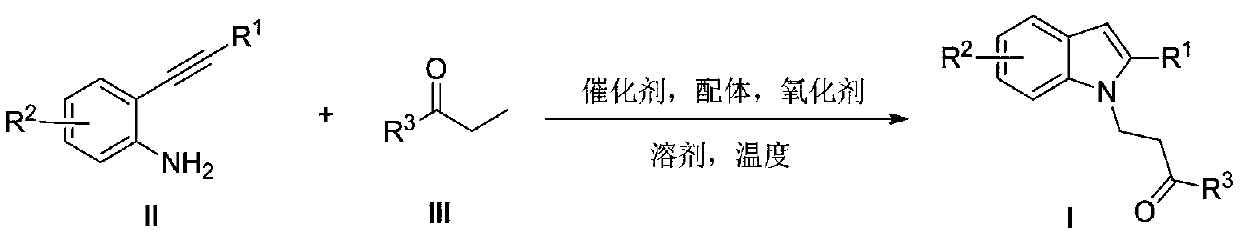
Preparation method of polysubstituted 2-aryl indole derivative
A technique for aryl indole derivatives and multi-substitution, which is applied in the field of preparation of multi-substituted 2-aryl indole derivatives, can solve the problems of limited substrate scope, unfavorable research, complex preparation and the like, and achieves easy operation and reaction. The effect of easy availability of raw materials, high chemoselectivity and regioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of 1-phenyl-3-(2-phenyl-1H-indol-1-yl)propan-1-one (Ia)
[0021] 2-Phenylethynylaniline (96.6 mg, 0.5 mmol), propiophenone (80.5 mg, 0.6 mmol), Cu(OAc) were added to a 10 mL reactor 2 (9.1mg, 0.05mmol), 2,2'-bipyridine (15.6mg, 0.1mmol), 4-hydroxy-2,2,4,4-tetramethylpiperidine nitroxide radical (4-OH-TEMPO ) (86.1 mg, 0.5 mmol) and 3 mL of 1,2-dichlorobenzene. After heating at 120°C for 24 hours, the solvent was recovered by concentration under reduced pressure and separated by column chromatography (n-hexane / ethyl acetate=50 / 1, v / v) to obtain a white solid 1-phenyl-3-(2-benzene (133.4 mg, 82%), melting point: 134-137°C. 1 H NMR (400MHz, CDCl 3 , ppm) δ7.76(d, J=7.2Hz, 2H), 7.65(d, J=8.0Hz, 1H), 7.60-7.38(m, 9H), 7.25(d, J=7.2Hz, 1H), 7.16(d,J=7.6Hz,1H),6.61(s,1H), 4.75-4.64(m,2H),3.33-3.23(m,2H). 13 C NMR (100MHz, CDCl 3 ,ppm) δ197.8,141.1,137.2,136.2,133.4,132.7,129.4,128.7,128.6,128.5, 128.2,128.0,121.9,120.7,120.2,109.8,102.9,39.3,38.5.H...
Embodiment 2
[0022] Example 2: Preparation of 1-phenyl-3-(2-phenyl-1H-indol-1-yl)propan-1-one (Ia)
[0023] 2-Phenylethynylaniline (96.6 mg, 0.5 mmol), propiophenone (80.5 mg, 0.6 mmol), Cu(OAc) were added to a 10 mL reactor 2 (9.1mg, 0.05mmol), 2,2'-bipyridine (15.6mg, 0.1mmol), 2,2,4,4-tetramethylpiperidine nitroxide (TEMPO) (78.1mg, 0.5mmol) and 3 mL of 1,2-dichlorobenzene. After heating at 120°C for 24 hours, the solvent was recovered by concentration under reduced pressure and separated by column chromatography (n-hexane / ethyl acetate=50 / 1, v / v) to obtain a white solid Ia (120.4 mg, 74%), melting point: 134~137℃.
Embodiment 3
[0024] Example 3: Preparation of 1-phenyl-3-(2-phenyl-1H-indol-1-yl)propan-1-one (Ia)
[0025] 2-Phenylethynylaniline (96.6 mg, 0.5 mmol), propiophenone (80.5 mg, 1.0 mmol), Cu(OAc) were added to a 10 mL reactor 2 (9.1 mg, 0.05 mmol), 2,2'-bipyridine (15.6 mg, 0.1 mmol), TEMPO (78.1 mg, 0.5 mmol) and 3 mL of chlorobenzene. After heating at 120°C for 24 hours, the solvent was recovered by concentration under reduced pressure and separated by column chromatography (n-hexane / ethyl acetate=50 / 1, v / v) to obtain a white solid Ia (71.6 mg, 44%), melting point: 134~137℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com