Wound dressing comprising hyaluronic acid-calcium and polylysine and manufacturing method therefor
A technology of hyaluronic acid and polylysine, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, and other household appliances to achieve excellent hemostatic function, adhesion, and fast blood absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
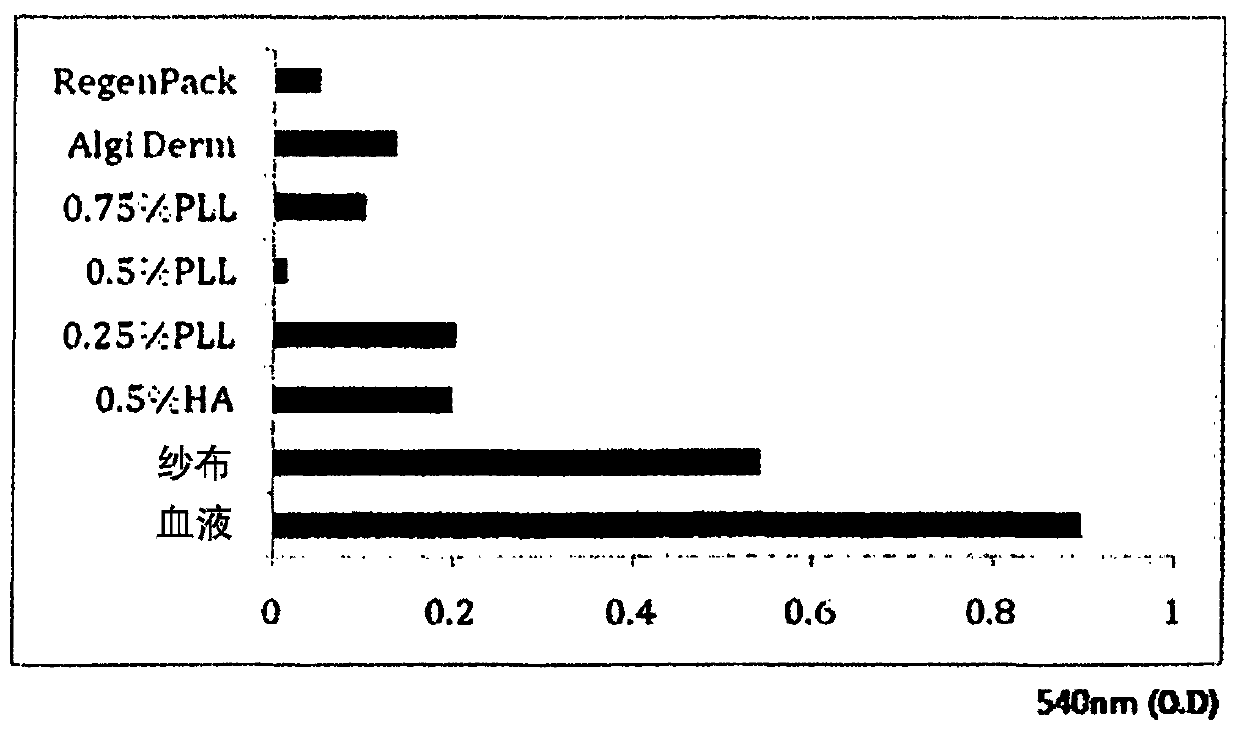
Image
Examples
Embodiment 1 to 6 and comparative example 1、2
[0066] Examples 1 to 6 and Comparative Examples 1 and 2: Manufacture of Foam Wound Covering (Patch)
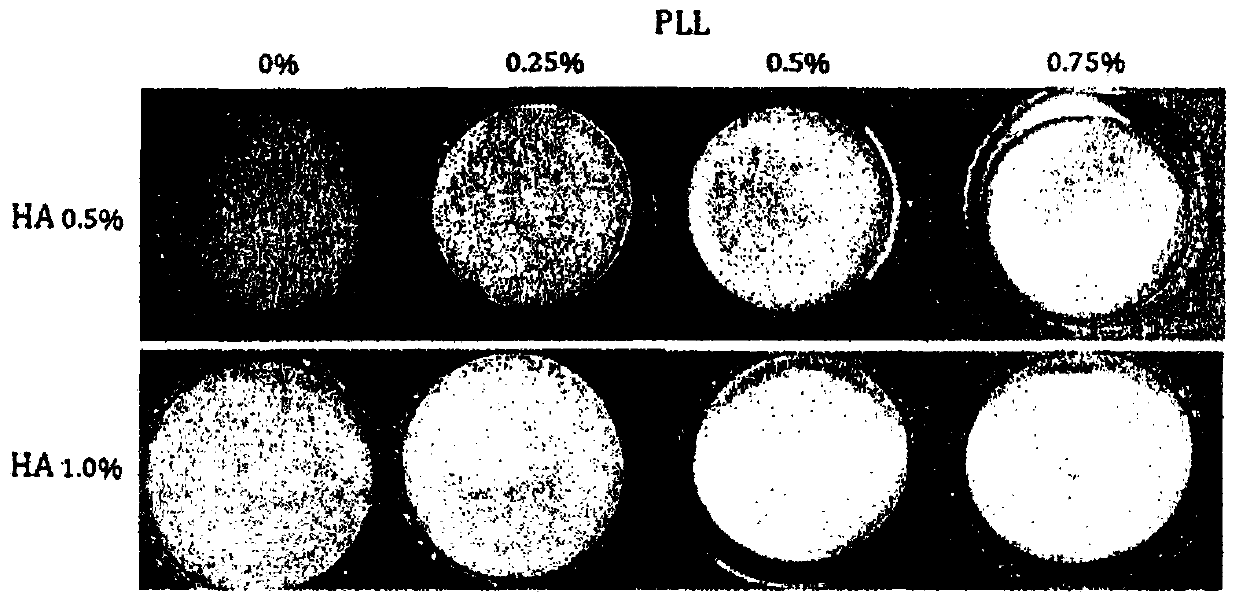
[0067] 5 g of hyaluronic acid was added to 450 ml of purified water, and stirred for 2 hours. 21.1 g of CaCl was dissolved in 50 ml of purified water, added to the above solution, and stirred slowly at room temperature for 3 hours to produce hyaluronic acid-calcium salt. After that, HA and PLL adjusted to pH 8.4, respectively, were mixed in the amounts shown in the following [Table 1] to prepare mixed liquids each having a total volume of 10 ml, the mixed liquids were allowed to stabilize at 0 to 4° C. for 6 to 10 hours, and then Freezing at -10 to -20°C for 10 to 12 hours, followed by freeze-drying, to manufacture HA-PLL foam-type wound coverings (hereinafter, also referred to as "HA-PLL patch" or "HA-Ca-PLL patch") sheet"), and observe whether foam is formed or not. The results are shown in Table 1 below. In Table 1, Comparative Examples 1 and 2 are patches made of only...
Embodiment 7
[0070] Embodiment 7: the manufacture of liquid type wound dressing
[0071] Sodium chloride was added to the mixed solution having the composition of Example 2 so as to have a concentration of 0.8%, and the resulting solution was charged into a prefilled syringe, thereby preparing a liquid-type wound covering.
Embodiment 8
[0072] Example 8: Manufacture of film-type wound dressing
[0073] The mixed liquid having the composition of Example 2 was poured into a mold with a size of 10 cm×10 cm, and placed in a thermostat at 40° C. for 12 to 16 hours to manufacture a film-type wound covering.
[0074] figure 1 These are photographs showing the appearance of the patches produced in Examples 1 to 6 and Comparative Examples 1 and 2. As above Table 1 and figure 1 As shown, the compositions of Examples 1 to 6 all formed patches well. However, the higher the HA content, the stiffer the patch was, showing a tendency to stiffen the patch even with increasing PLL content. On the other hand, when the PLL content in the production liquid exceeds 0.75% (w / v) or the HA content exceeds 1% (w / v), it becomes brittle due to too high hardness. Therefore, in the case of providing the wound dressing in a foam type or a film type, an HA content of not more than 1.0% and a PLL content of not more than 0.75% are evalua...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com