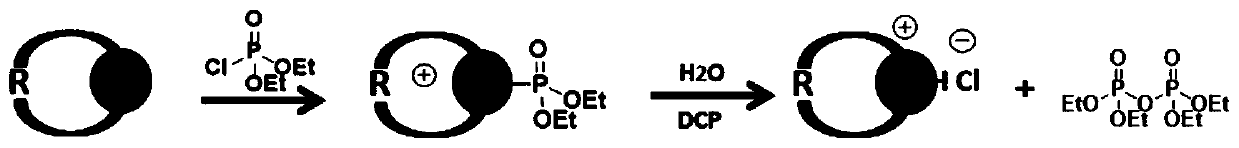
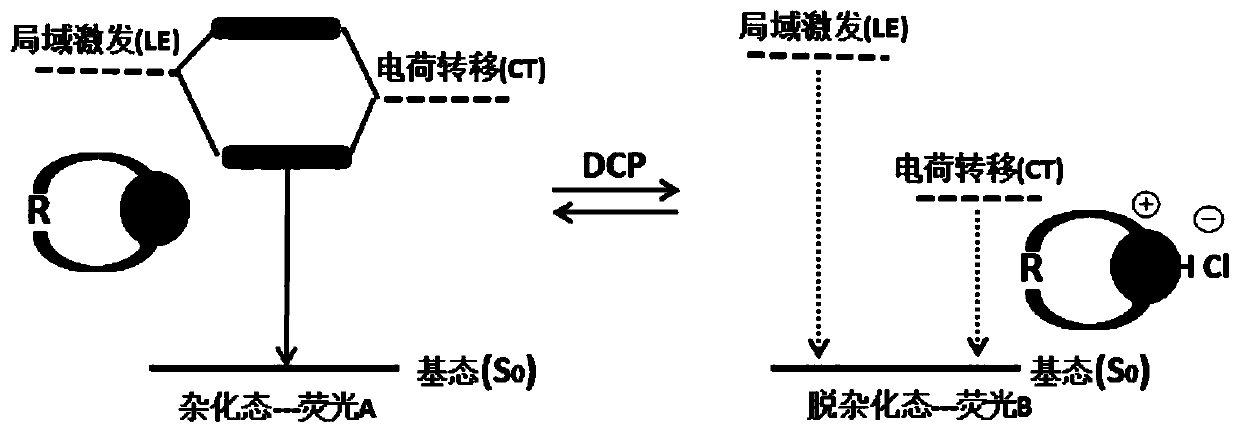
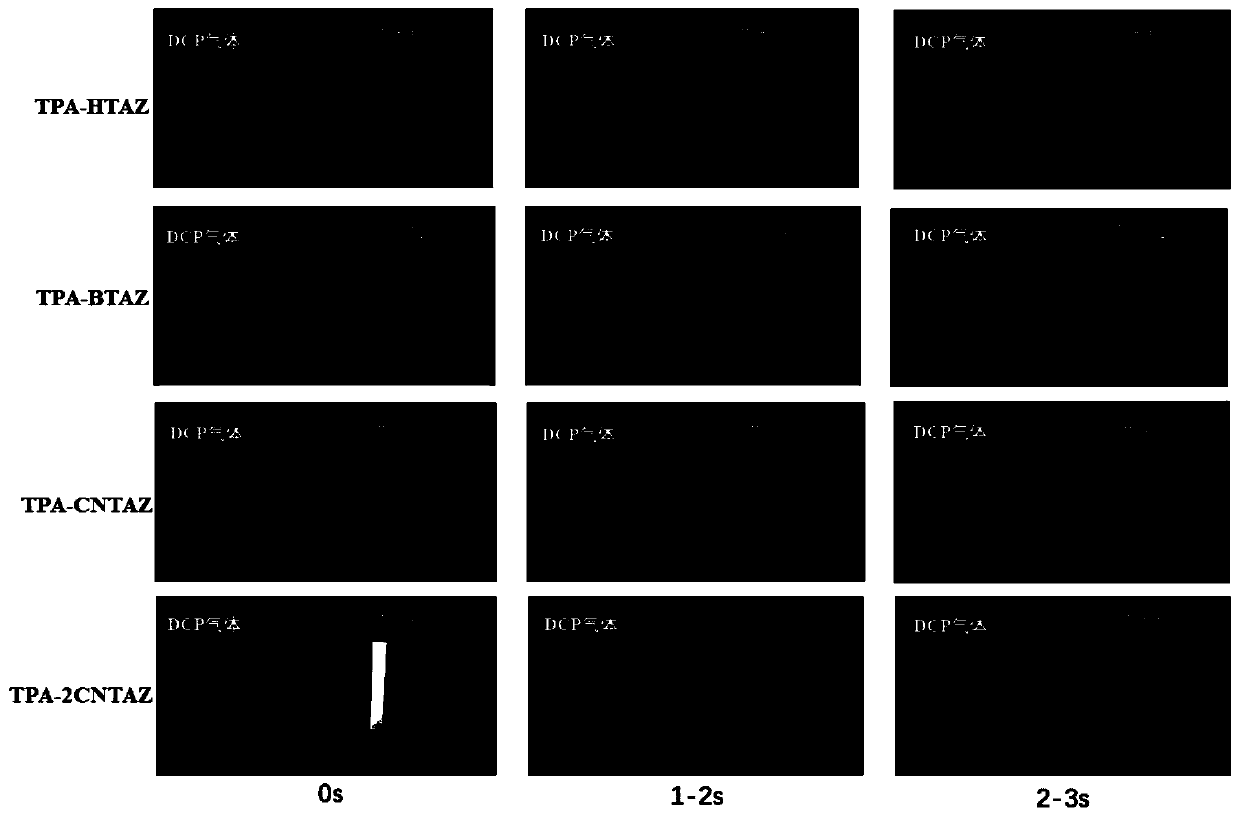
Fluorescent probes and application thereof to detection of neurotoxic agents
A fluorescent probe and nerve agent technology, applied in the field of fluorescent probes, can solve the problems that the detection limit of fluorescent probes is difficult to meet the needs of practical applications, and the substrate selectivity is limited, so as to achieve rapid nerve agent response and specific recognition , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0038] Synthesis of Preparation Example 1 TPA-HTAZ (Fluorescent Probe 1)
[0039] Step (1): Synthesis of the intermediate bromobenzoyl hydrazide (Br-2O-Br)
[0040] Weigh 4-bromobenzoyl chloride, hydrazine hydrate and triethylamine in a molar ratio of about 2-3:1:2-3. At 0°C, dissolve 4-bromobenzoyl chloride in chloroform. After it is completely dissolved, add triethylamine with a dropper, and then slowly add hydrazine hydrate into the mixed solution with a syringe. It can be observed that a white precipitate appears, stirred under this condition for 0.5-1 hour, then returned to room temperature and continued to stir for 3-5 hours. After the reaction, the product is suction-filtered, thoroughly washed with petroleum ether and deionized water, and dried. The structural characterization is as follows: Mass Spectrum: MW 398.05, m / z=398.05 (M+). 1 H NMR (500 MHz, DMSO) δ 10.64 (s, 2H), 7.87 (d, J = 8.6 Hz, 4H), 7.76 (d, J = 8.5 Hz, 4H).
[0041]
[0042] Step (2): Synthesis...
preparation Embodiment 2
[0051] Synthesis of Preparation Example 2 TPA-BTAZ (fluorescent probe 2)
[0052] The preparation of step (1) and (2) intermediate product is respectively the same as step (1) and (2) among the embodiment 1.
[0053] Step (3): Synthesis of intermediate product Br-TAZ-B
[0054] The chloroazobromotoluene and aniline were weighed in a molar ratio of 1:1-1.8. Dissolve the weighed raw materials in degassed N-methylpyrrolidone (or N,N-dimethylformamide), and stir for 10-14 hours under nitrogen protection at 130-140°C under anhydrous and oxygen-free conditions. After cooling, add about 10-15mL of 2M dilute hydrochloric acid, and a precipitate can be observed during the dropwise addition of dilute hydrochloric acid. After continuing to stir at room temperature for 30-60 minutes, the product is suction filtered, washed with a large amount of deionized water, and dried. The structural characterization is as follows: Mass Spectrum: MW454.97, m / z=454.95 (M+). 1 H NMR (500MHz, DMSO) δ...
preparation Embodiment 3
[0058] Synthesis of Preparation Example 3 TPA-CNTAZ (fluorescent probe 3)
[0059] The synthesis of step (1) and (2) intermediate product is respectively the same as step (1) and (2) among the embodiment 1.
[0060] Step (3): Synthesis of intermediate product Br-TAZ-CN
[0061] Weigh chloroazobromotoluene and 4-aminobenzonitrile at a molar ratio of 1:1-1.8. Dissolve the weighed raw materials in degassed N-methylpyrrolidone (or N,N-dimethylformamide), and stir for 10-14 hours under nitrogen protection at 130-140°C under anhydrous and oxygen-free conditions. After cooling, add about 10-15mL of 2M dilute hydrochloric acid, and a precipitate can be observed during the dropwise addition of dilute hydrochloric acid. After continuing to stir at room temperature for 30-60 minutes, the product is suction filtered, washed with a large amount of deionized water, and dried. Mass spectrum: MW808.67, m / z=808.99 (M+). 1 H NMR (500MHz, Chloroform-d) δ7.81–7.75(m,2H),7.60–7.53(m,4H),7.52–7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com