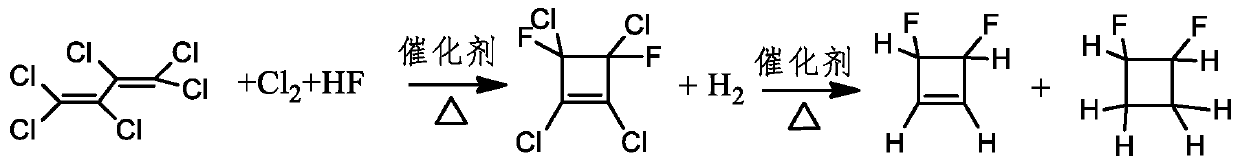
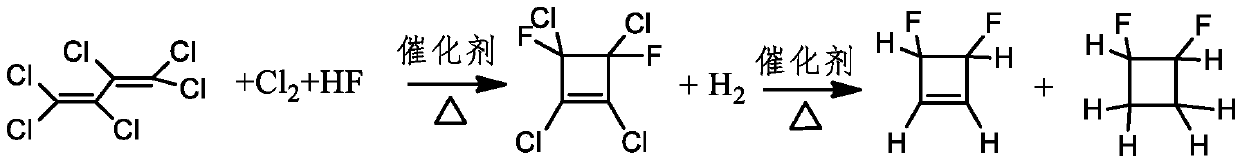
Method for gas phase catalytic synthesis of 3,4-difluorocyclobutene
A technology of difluorocyclobutene and gas phase, which is applied in the field of gas phase catalytic synthesis of 3,4-difluorocyclobutene, which can solve the problems of expensive raw materials, harsh conditions, and long routes, and achieve convenient sources, safe production, and raw material sources wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) adopt co-precipitation method to prepare ring fluorination catalyst, the steps are as follows:
[0027] CrCl with a molar ratio of 65:20:15 3 , Co(NO 3 ) 2 , Zn(NO 3 ) 2 The solutions were mixed, and 30 wt% ammonia water was added dropwise to the mixed solution to adjust the pH to 10.0. Precipitation and filtration, washing with deionized water, drying, and pressing to obtain the ring fluorination catalyst precursor Cr-Co-Zn;
[0028] Put 50ml of ring fluorination catalyst Cr-Co-Zn precursor into the fixed bed reactor, and the fixed bed reactor is heated by an open tube heating furnace. Under the protection of 100ml / min nitrogen, the catalyst was first dried at a rate of 1°C / min to 400°C for 10 hours, and then the temperature was lowered to 200°C. This completes the drying process of the ring fluorination catalyst.
[0029] Heat the reactor to 300°C, activate the catalyst with 100ml / min nitrogen and 20ml / min hydrogen fluoride for 10 hours; activate the cataly...
Embodiment 2
[0037] (1) adopt co-precipitation method to prepare ring fluorination catalyst, the steps are as follows:
[0038] CrCl with a molar ratio of 80:10:10 3 , Co(NO 3 ) 2 , Fe(NO 3 ) 3 The solutions were mixed, and 30 wt% ammonia water was added dropwise to the mixed solution to adjust the pH to 8.0. Precipitation and filtration, washing with deionized water, drying, and pressing to obtain the ring fluorination catalyst precursor Cr-Co-Fe;
[0039] Put 50ml of ring fluorination catalyst Cr-Co-Fe precursor into the fixed bed reactor, and the fixed bed reactor is heated by an open tube heating furnace. Under the protection of 100ml / min nitrogen, the catalyst was first dried at a rate of 1°C / min to 400°C for 10 hours, and then the temperature was lowered to 200°C. This completes the drying process of the ring fluorination catalyst.
[0040] Heat the reactor to 300°C, activate the catalyst with 100ml / min nitrogen and 20ml / min hydrogen fluoride for 10 hours; activate the catalys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com