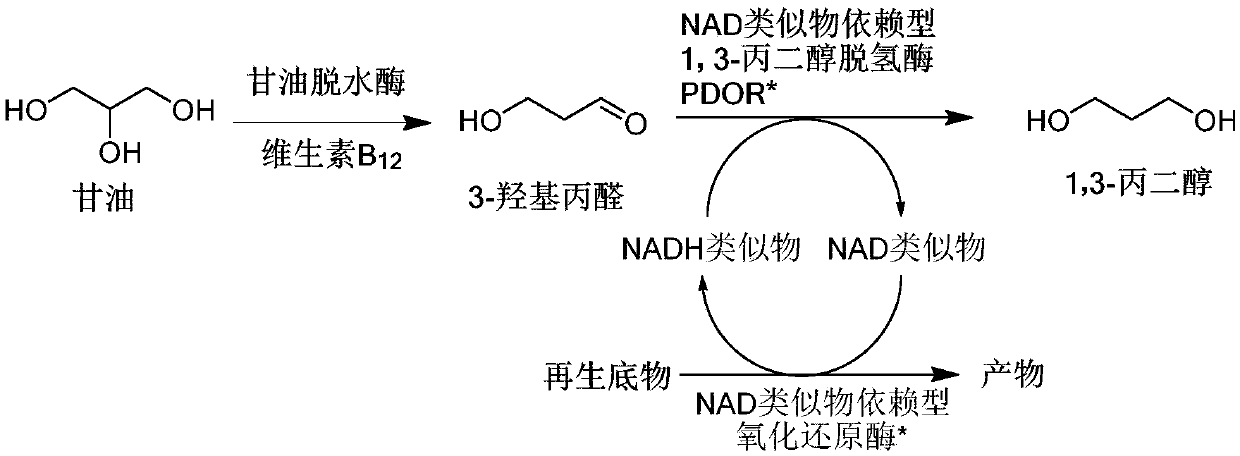
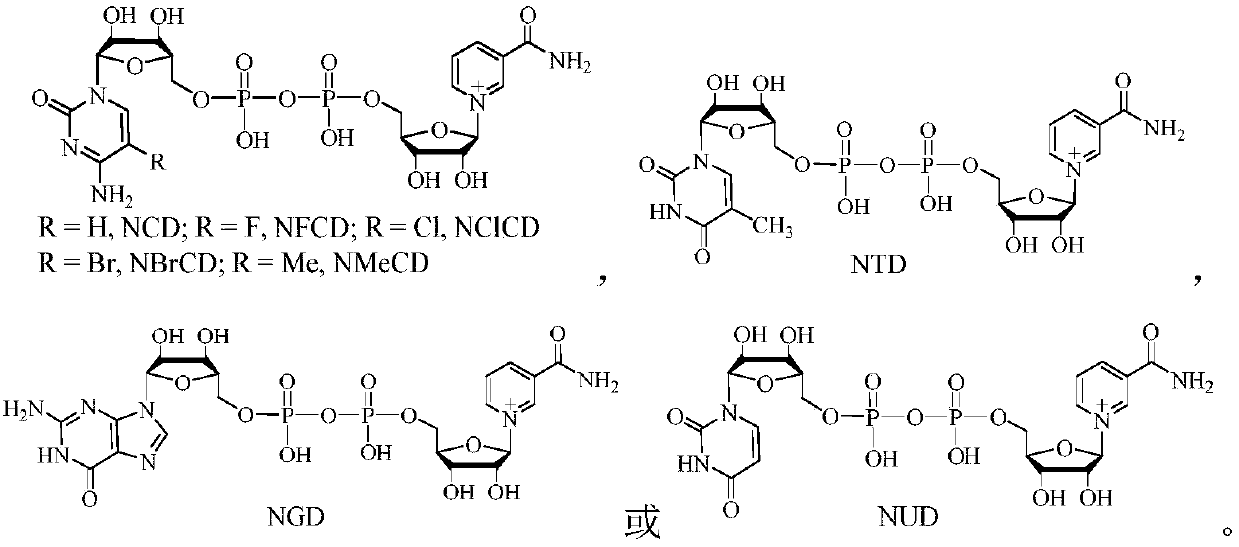
Method for producing 1,3-propanediol by biologically catalyzing glycerol
A technology for biologically catalyzing glycerol and propylene glycol, which is applied in the directions of biochemical equipment and methods, botanical equipment and methods, oxidoreductases, etc., and can solve problems such as affecting the utilization efficiency of reducing power, improving, and difficulty in 1,3-propanediol production. , to achieve the effect of improving product selectivity, high reaction efficiency and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Using glycerol as raw material, 1,3-propanediol was prepared under the catalysis of wild-type glycerol dehydratase and PDOR-T143I / G186P.
[0054] According to the literature method (Fuller, C.W. et al. Eur. J. Biochem. 1980, 103, 421), NCD was reduced to NCDH, which was used as a reducing agent for 3-hydroxypropanal.
[0055] In 1mL of HEPES buffer reaction system containing 50mM pH7.5, containing 30mM (NH 4 ) 2 SO 4 , 100mM glycerol, 110mM NCDH, derived from Klebsiella pneumoniae glycerol dehydratase GDHt15.8μg / mL, NCD-dependent 1,3-propanediol dehydrogenase PDOR-T143I / G186P 18.9μg / mL, added 2mM vitamin B 12 Start the reaction, react at 37°C for 2 hours, add 9 mL of acetonitrile methanol water mixture (acetonitrile: methanol: water volume ratio = 4:4:1) to quench the reaction, take out 500 μL for coenzyme and product analysis.
[0056] HPLC analysis: the sample has NCDH characteristic peak at 340nm, the content is 40.5%, indicating that NADH is not comple...
Embodiment 2
[0059] Example 2: Using glycerol as raw material, under the catalysis of wild-type glycerol dehydratase and PDOR-G43K / G186P / L191F body, sodium phosphite is used as NCD analog recycling reagent to prepare 1,3-propanediol
[0060] In 1mL of HEPES buffer reaction system containing 50mM pH7.5, containing 30mM (NH 4 ) 2 SO 4 , 100mM glycerol, 2mM NCD, 120mM sodium phosphite, derived from Klebsiella pneumoniae wild-type glycerol dehydratase GDHt15.8μg / mL, PDOR-G43K / G186P / L191F 17.9μg / mL, phosphite dehydrogenase rsPDH-I151R / E213C 10U / mg, add 2mM vitamin B 12 Start the reaction, react at 37 ° C for 180 min, add 9 mL of acetonitrile methanol water mixture (acetonitrile: methanol: water = 4:4:1) to quench the reaction, take out 500 μL for coenzyme and product analysis.
[0061] HPLC analysis: the sample has a characteristic peak at 340nm, and the NCDH content is 12.9%, and a characteristic peak with the same retention time as NAD is detected at 260nm, and the content is 87.1%, indic...
Embodiment 3
[0067] Example 3: Using glycerol as a raw material, under the catalysis of wild-type glycerol dehydratase and PDOR-G43D / L44F / G186P / L191Y, potassium deuterated phosphite is used as a NUD analogue recycling reagent to prepare 1,3-propanediol
[0068] According to literature method (Woodyer R, et al. FEBS J, 2005, 272, 3816), potassium phosphite was dissolved in D 2 O, freeze-dried, repeated 4 times to obtain deuterated potassium phosphite, and set aside.
[0069] In 5 mL of Tris-HCl buffer reaction system containing 100 mM pH 8.5, containing 30 mM (NH 4 ) 2 SO 4 , 100mM glycerol, 2mM NUD, 120mM potassium deuterophosphite, derived from Klebsiella aerogenes wild-type glycerol dehydratase GDHt 15.8μg / mL, PDOR-G43K / G186P / L191F 18.9μg / mL, phosphorous acid dehydrogenation Enzyme psPDH-L151V / D213Q 20μg / mL, add 5mM Vitamin B 12 Start the reaction, react at 37°C for 30 min, add 45 mL of acetonitrile methanol water mixture (acetonitrile: methanol: water = 4:4:1) to quench the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com