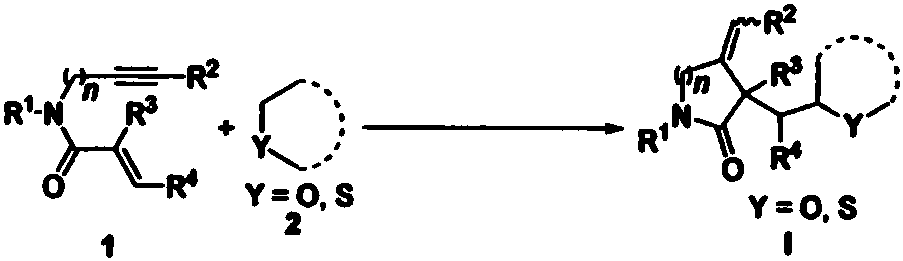
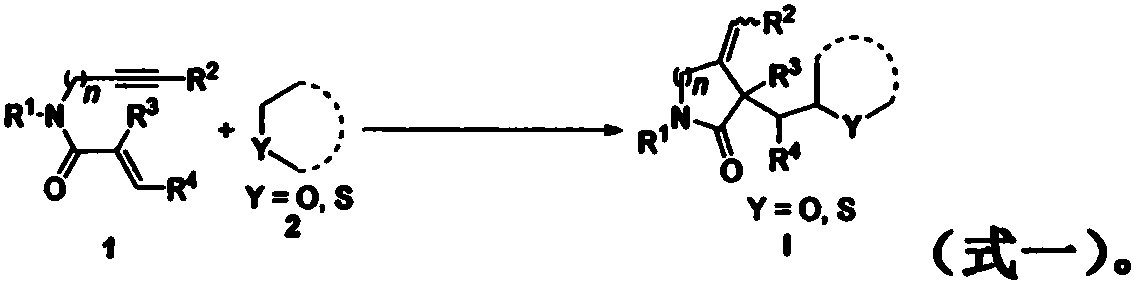
Free radical cyclization reaction method of 1,6-eneyne compound and ether compound
A technology of ether compounds and cyclization reaction, applied in the direction of organic chemistry, etc., can solve the problems of limiting the diversity of organic molecules, and achieve the effect of adapting to a wide range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Add 1,6-enyne compound represented by formula 1a (39.8 mg, 0.2 mmol), tetrahydrofuran (0.5 mL), tert-butyl peroxybenzoate (TBPB, 155.2 mg, 0.8 mmol), cesium carbonate to a Schlenk bottle (Cs 2 CO 3, 78.2mg, 0.24mmol), then the reactor was stirred and reacted in an air atmosphere at 85°C, and the reaction process was monitored by TLC until the raw material disappeared (reaction time was 20 hours). After the reaction was completed, the reaction solution was concentrated under reduced pressure to remove Solvent, the residue was separated by column chromatography (elution solvent: ethyl acetate / n-hexane) to obtain the target product I-1 (78% yield, d.r.=5.7:1); 1 H NMR (400MHz, DMSO-d6) δ: 7.74 (d, J=8.4Hz, 0.3H), 7.69 (d, J=8.0Hz, 1.7H), 7.42-7.36 (m, 2H), 7.17-7.12( m, 1H), 5.29-5.02(m, 2H), 4.51(d, J=14.4Hz, 1H), 4.38(d, J=16.0Hz, 1H), 3.90-3.84(m, 1H), 3.60-3.54 (m, 1H), 3.45-3.40(m, 1H), 2.01-1.95(m, 1H), 1.89-1.82(m, 1H), 1.78-1.63(m, 3H), 1.44-1.37(m, ...
Embodiment 2
[0038] No oxidizing agent was added, other conditions were the same as in Example 1, and the yield of the target product I-1 was 0%.
Embodiment 3
[0040] No base was added, and the other conditions were the same as in Example 1, and the yield of the target product I-1 was 23%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com