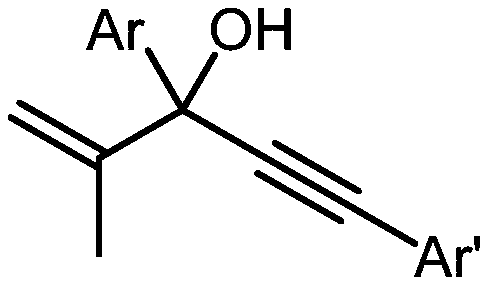
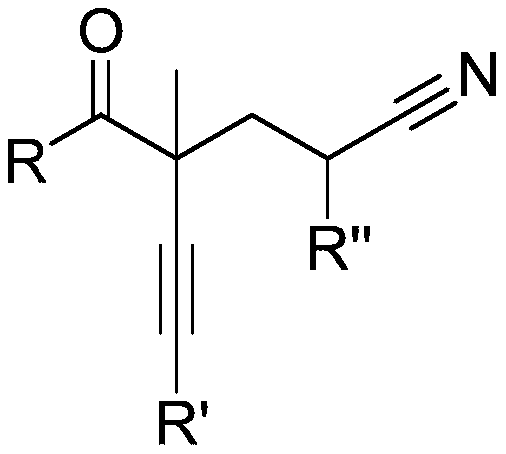
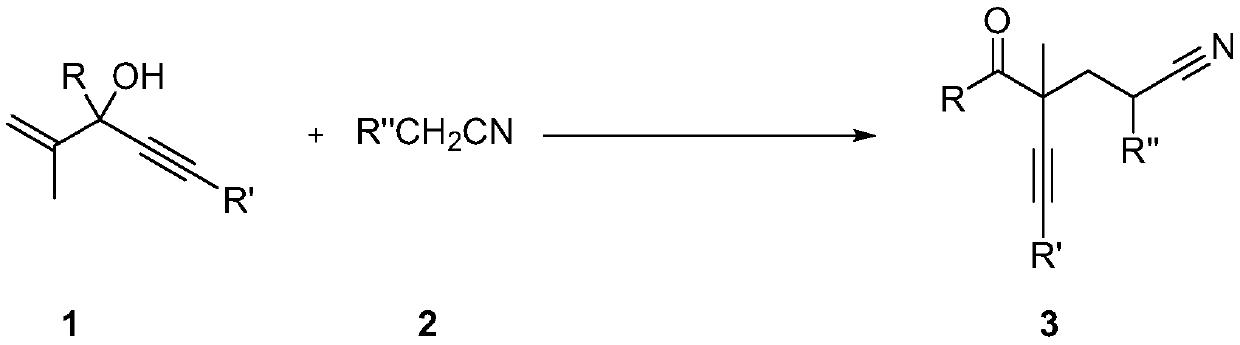
Method for preparing alpha-alkynyl gamma-cyano functionalized ketones from allyl alcohol
A technology of cyano function and allyl alcohol, which is applied in the field of difunctionalization reaction of free radical process, can solve the problems of expensive transition metals and ligands, metal residues in products, etc., and achieves a wide range of substrates and mild reaction conditions , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of 4-benzoyl-4-methyl-6-phenyl-5-hexynenitrile
[0031] [4-benzoyl-4-methyl-6-phenylhex-5-ynenitrile]
[0032]
[0033] In the dried Schlenk tube, add in sequence: 2-methyl-3,5-diphenyl-1-en-4-yn-3-ol (0.2mmol, 49.6mg), di-tert-butyl Peroxide (0.6mmol, 87.6mg), acetonitrile 3mL, and the above-mentioned Schlenk tube was stirred at a temperature of 120°C for 12h. The reaction was terminated, and the solvent was removed on a rotary evaporator. Finally, it was separated by silica gel column chromatography (eluent: ethyl acetate: petroleum ether = 1:10) to obtain 4-benzoyl-4-methyl-6-phenyl-5-hexynenitrile (38.5mg , Isolation yield: 67%), the compound is a yellow oil.
[0034] NMR data: 1 H NMR (CDCl 3 ,400MHz):δ8.34-8.32(m,2H),7.59-7.56(m,1H),7.49-7.45(m,2H),7.40-7.29(m,5H),2.67-2.58(m,3H) ,2.10-2.03(m,1H),1.72(s,3H); 13 C NMR (100MHz, CDCl 3 )δ197.5, 134.5, 133.1, 131.3, 129.7, 128.6, 128.3, 128.1, 122.1, 119.7, 89.4, 87.8, 45.8, 35.2, ...
Embodiment 2
[0035] Embodiment 2: Preparation of 4-benzoyl-4-methyl-6-(4-propylphenyl)-5-hexynenitrile
[0036] [4-benzoyl-4-methyl-6-(4-propylphenyl)hex-5-ynenitrile]
[0037]
[0038]In the dried Schlenk tube, add successively: 2-methyl-3-phenyl-5-(4-propylphenyl)pent-1-en-4-yn-3-ol (0.2mmol , 58mg), di-tert-butyl peroxide (0.6mmol, 87.6mg), acetonitrile 3mL, and the above-mentioned Schlenk tube was stirred at a temperature of 120°C for 12h. The reaction was terminated, and the solvent was removed on a rotary evaporator. Finally, it was separated by silica gel column chromatography (eluent: ethyl acetate: petroleum ether = 1:10) to obtain 4-benzoyl-4-methyl-6-(4-propylphenyl)-5- Hexynenitrile (36.8 mg, isolated yield: 56%), the compound was a yellow oil.
[0039] NMR data: 1 H NMR (CDCl 3 ,400MHz):δ8.34-8.32(m,2H),7.59-7.55(m,1H),7.48-7.44(m,2H),7.31-7.29(m,2H),7.14-7.12(m,2H) ,2.67-2.56(m,5H),2.08-2.01(m,1H),1.71(s,3H),1.67-1.58(m,2H),0.95-0.91(m,3H); 13 C NMR (100MHz, CDCl 3...
Embodiment 3
[0040] Example 3: Preparation of 4-benzoyl-4-methyl-6-(4-p-tolyl)-5-hexynenitrile
[0041] [4-benzoyl-4-methyl-6-(4-propylphenyl)hex-5-ynenitrile]
[0042]
[0043] In the dried Schlenk tube, add successively: 2-methyl-3-phenyl-5-(p-tolyl)-1-en-4-yn-3-ol (0.2mmol, 52.4mg) , di-tert-butyl peroxide (0.6mmol, 87.6mg), acetonitrile 3mL, and the above-mentioned Schlenk tube was stirred at a temperature of 120°C for 12h. The reaction was terminated, and the solvent was removed on a rotary evaporator. Finally, it was separated by silica gel column chromatography (eluent: ethyl acetate: petroleum ether = 1:10) to obtain 4-benzoyl-4-methyl-6-(4-p-tolyl)-5-hexane Alkynenitrile (36.8 mg, isolated yield: 56%), the compound was a yellow oil.
[0044] NMR data: 1 H NMR (CDCl 3 ,400MHz):δ8.34-8.32(m,2H),7.59-7.55(m,1H),7.48-7.44(m,2H),7.29-7.27(m,2H),7.13-7.11(m,2H) ,2.69-2.57(m,3H),2.35(s,3H),2.09-2.02(m,1H),1.71(s,3H); 13 C NMR (100MHz, CDCl 3 )δ197.6, 138.8, 134.5, 133.0, 131.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com