Salt-tolerant xylosidase mutant T326DH328D as well as preparation and application thereof
A technology of T326DH328D and xylosidase, which is applied in the directions of glycosylase, botanical equipment and methods, biochemical equipment and methods, etc., can solve problems such as lack of stability, and achieve the effect of enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0029] Construction and Transformation of Experimental Example 1 Expression Vector
[0030] According to the xylosidase nucleotide sequence KY391885 (SEQ ID NO.4) recorded in GenBank, the gene hJ14GH43 encoding the wild xylosidase HJ14GH43 was synthesized; the gene t326dh328d (SEQ ID NO.2) encoding the mutant enzyme T326DH328D was also synthesized.
[0031] The synthetic xylosidase nucleotide and mutant enzyme T326DH328D nucleotide sequences were connected to the expression vector pEasy-E1 to obtain the expression vectors containing hJ14GH43 and t326dh328d, and the ligated products were transformed into Escherichia coli BL21 (DE3) to obtain the respective Recombinant strain expressing wild enzyme HJ14GH43 and mutant enzyme T326DH328D.
experiment example 2
[0032] Experimental Example 2 Preparation of Wild Enzyme HJ14GH43 and Mutant Enzyme T326DH328D
[0033] The recombinant strains containing hJ14GH43 and t326dh328d were inoculated in LB (containing 100 μg mL -1 Amp) medium, shake rapidly at 37°C for 16h.
[0034] Then, the activated bacterial solution was inoculated into fresh LB (containing 100 μg mL -1 Amp) culture medium, rapid shaking culture for about 2 ~ 3h (OD 600 After reaching 0.6-1.0), add IPTG at a final concentration of 0.1 mM for induction, and continue shaking culture at 20° C. for about 20 h.
[0035] Centrifuge at 12000rpm for 5min to collect the bacteria. Suspend the bacteria with an appropriate amount of pH 7.0 Tris-HCl buffer solution, and then ultrasonically break the bacteria in a low-temperature water bath.
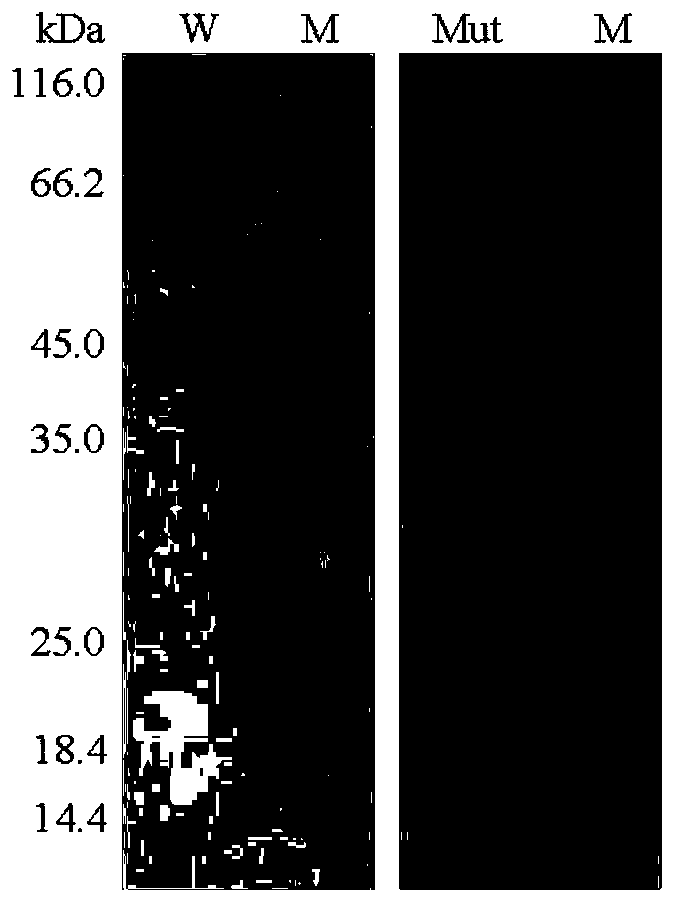
[0036] After the crude enzyme solution concentrated in the cells was centrifuged at 12,000rpm for 10min, the supernatant was aspirated and the target protein was affinity and eluted with Nickel-NT...
experiment example 3
[0038] Determination of the properties of the purified wild enzyme HJ14GH43 and mutant enzyme T326DH328D of Experimental Example 3
[0039] The activities of the purified wild enzyme HJ14GH43 and the mutant enzyme T326DH328D were determined by the pNP method, as follows:
[0040] Dissolve pNPX in the buffer solution to make the final concentration 2mM; the reaction system contains 50μL of appropriate enzyme solution and 450μL of 2mM substrate; after the substrate is preheated at the reaction temperature for 5min, add the enzyme solution and react for an appropriate time, then add 2mL 1M Na 2 CO 3 The reaction was terminated, and the released pNP was measured at a wavelength of 405 nm after cooling to room temperature; 1 enzyme activity unit (U) was defined as the amount of enzyme required to decompose the substrate to produce 1 μmol pNP per minute.
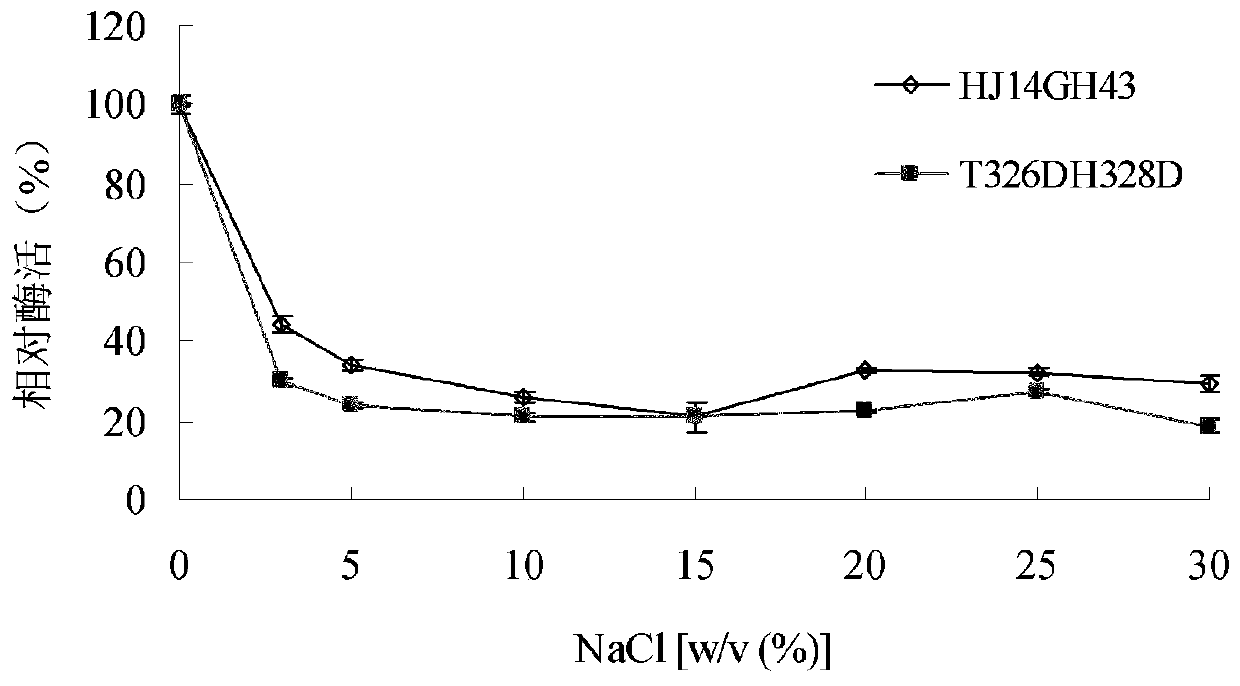
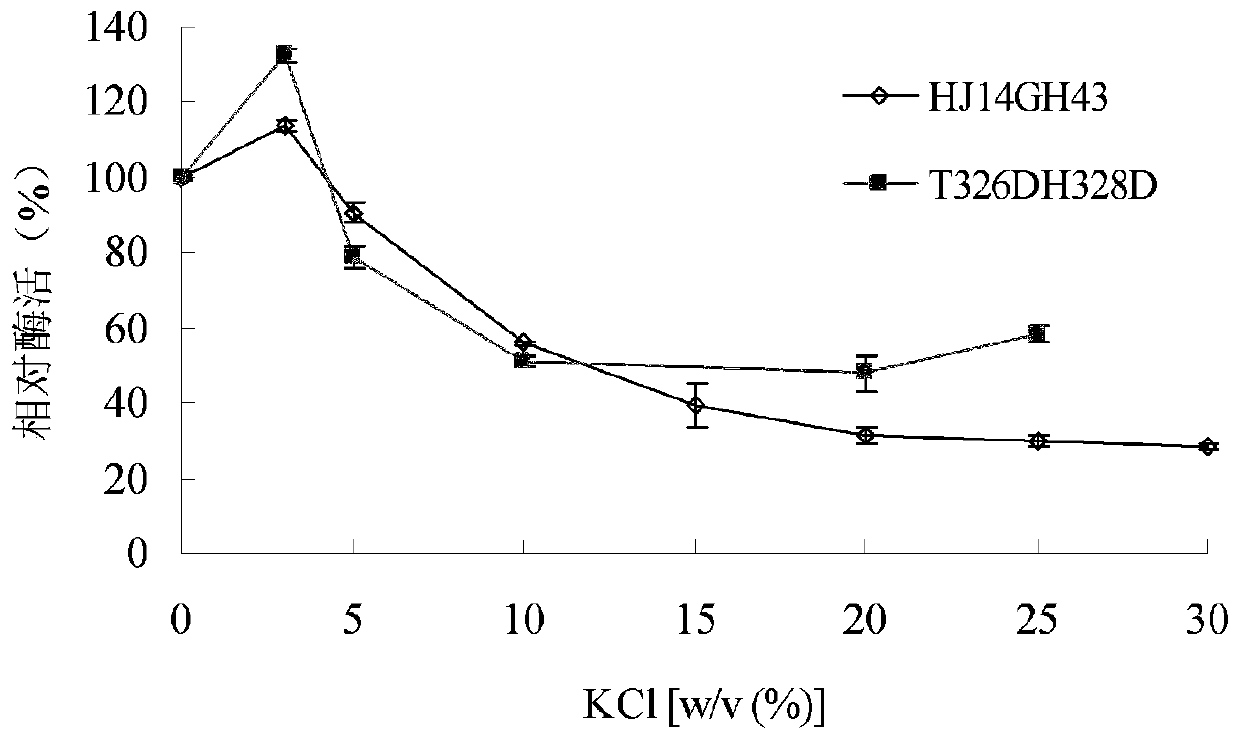
[0041] 1. Stability of purified wild enzyme HJ14GH43 and mutant enzyme T326DH328D in NaCl
[0042] The purified enzyme soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com