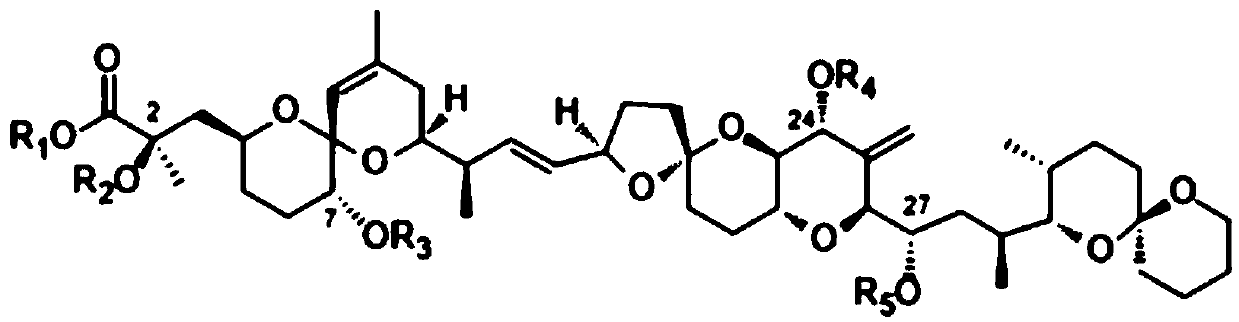
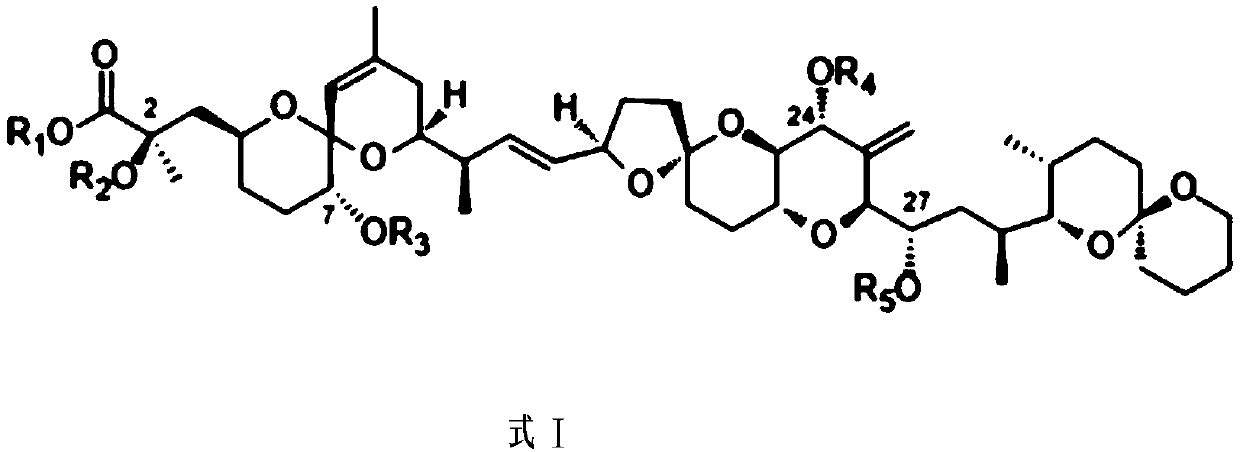
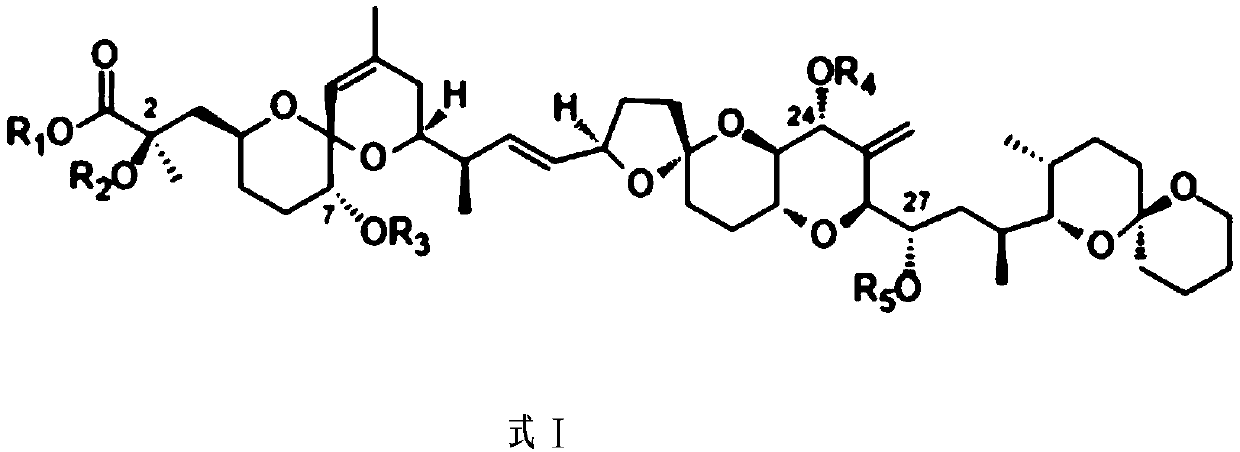
Okadaic acid derivative and preparation method thereof
A technology of Datian soft sponge acid and derivatives, applied in the direction of organic chemistry, etc., can solve the problems of low total yield, long synthesis route and high difficulty, and achieve the effects of reducing the generation of by-products, fewer processes, and broad market prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Benzyl esterification of okadaic acid to prepare compound 1;
[0028] Procedure: Add 2.4mg okadaic acid, 5mg K to a 25mL round bottom flask 2 CO 3 and 4 mL of anhydrous acetone, take 2 drops of benzyl bromide and dissolve in 1 mL of anhydrous acetone in a constant pressure dropping funnel, stir in an ice-water bath for 1 hour, then slowly drop into the reaction flask; stir overnight at room temperature. Add 5 mL of ice water, neutralize with 0.5M HCl, extract with ethyl acetate (3 mL×3), and wash with saturated brine. The organic layers were combined and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the crude product was subjected to flash silica gel column chromatography (chloroform:methanol=30:1) to obtain 2.3 mg of compound 1: light brown amorphous solid, yield 86%.
Embodiment 2
[0030] Compound 2 was prepared by benzoylation of okadaic acid;
[0031] Procedure: 4.8 mg of okadaic acid, 2 drops of benzoyl chloride, 1 mg of DMAP and 2 mL of anhydrous pyridine were added to a 25 mL round bottom flask. Stir overnight at room temperature. Add 5 mL of ice water, extract with ethyl acetate (3 mL×3), wash with 1M HCl, and wash with saturated brine. The organic layers were combined and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the crude product was subjected to flash silica gel column chromatography (chloroform:methanol=60:1) to obtain 4.8 mg of compound 2: light brown amorphous solid, yield 66%.
Embodiment 3
[0033] Propionylation of okadaic acid to prepare compound 3;
[0034] Procedure: 2.4 mg of okadaic acid, 2 drops of propionic anhydride, 0.5 mg of DMAP and 2 mL of anhydrous pyridine were added to a 25 mL round bottom flask. Stir overnight at room temperature. Add 5 mL of ice water, extract with ethyl acetate (3 mL×3), wash with 1M HCl, and wash with saturated brine. The organic layers were combined and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the crude product was subjected to flash silica gel column chromatography (chloroform:methanol=60:1) to obtain 2.2 mg of compound 3: a white amorphous solid, with a yield of 72%.
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com