Substituted quinazoline compound, pharmaceutical composition containing substituted quinazoline compound, and applications of substituted quinazoline compound
A compound and application technology, applied in the field of substituted quinazoline compounds, can solve the problem of not being able to reach tumor cells, etc., and achieve the effects of good pharmacodynamic performance, high inhibitory activity, and excellent brain barrier penetration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
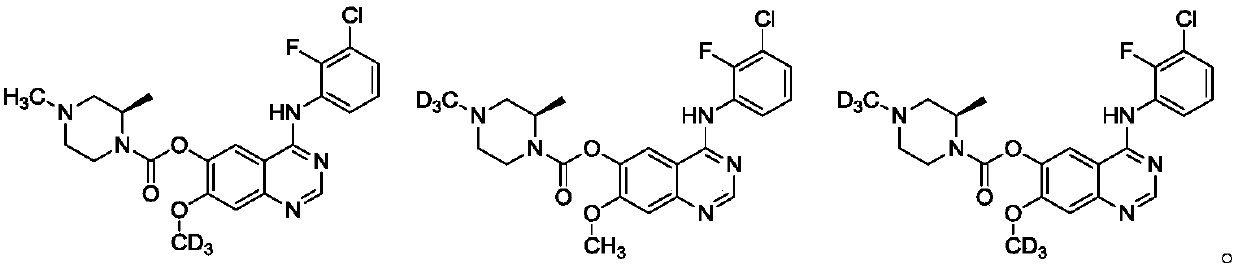
[0117] 4-[(3-Chloro-2-fluorophenyl)amino]-7-deuteromethoxyquinazolin-6-yl-(2R)-2,4-dimethylpiperazine-1-carboxylic acid ester
[0118]
[0119] Intermediate H (8.1 g, 16 mmol, 87% purity), paraformaldehyde (1 g, 32 mmol) triethylamine (1.61 g, 16 mol) were dissolved in methanol (100 mL), and sodium cyanoborohydride (2 g , 32mmol), the reaction solution was stirred at room temperature for 10 hours, after the reaction was completed, the solvent was evaporated, the residue was added with water, extracted with ethyl acetate (3×100mL), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain The crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate) to obtain the target product Example 1 (5.5 g). 1 H-NMR (400MHz, CDCl 3 )δ: 8.78(s,1H), 8.54-8.49(m,1H), 7.64(s,1H), 7.42(br,1H), 7.34(s,1H), 7.20-7.16(m,2H), 4.51 -4.49(m,1H),4.22-4.03(m,1H),3.52-3...
Embodiment 1A
[0121] 4-[(3-Chloro-2-fluorophenyl)amino]-7-deuteromethoxyquinazolin-6-yl-(2R)-2,4-dimethylpiperazine-1-carboxylic acid Ester hydrochloride
[0122]
[0123] Example 1 (3.6g) was dissolved in acetonitrile (10mL), and 1N HCl (10mL) was slowly added under stirring. After stirring for a while, the solvent was removed by lyophilization to obtain Example 1A (3.85g) as a yellow solid. 1 H-NMR (400MHz,D 2 O)δ: 8.56(s,1H),8.32-8.14(m,1H),7.51(m,1H),7.40(m,1H),7.31(s,1H),7.25-7.16(m,3H), 4.75-4.53(m,1H),4.50-4.13(m,1H),4.09-3.96(m,3H),3.75-3.49(m,1H),3.25-3.13(m,1H),2.32(s,3H ),1.50(br,3H); MS m / z463.2(M+1) + .
Embodiment 2
[0125] 4-[(3-Chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl-(2R)-2-methyl-4-deuteromethylpiperazine-1- Carboxylate
[0126]
[0127] Intermediate G (8.2g, 16mmol) and sodium hydride (24mmol) were dissolved in tetrahydrofuran (150mL), cooled to 0°C and stirred, and a solution of deuteromethyl iodide (3.5g, 24mmol) in tetrahydrofuran (20mL) was slowly added dropwise, During the dropwise addition process, the temperature of the reaction system was controlled between 0-5°C. After the reaction, add aqueous ammonium chloride solution (300mL) and stir, extract with ethyl acetate (3×200mL), dry over anhydrous sodium sulfate, and concentrate to obtain the crude product. Silica gel Purified by column chromatography (petroleum ether: ethyl acetate) to obtain the target product Example 2 (5.2 g). 1 H-NMR (400MHz, CD 3 OD)δ: 8.77(s,1H),8.55-8.50(m,1H),7.66(s,1H),7.43(br,1H),7.35(s,1H),7.19-7.16(m,2H), 4.52-4.51(m,1H),4.19-4.04(m,1H),3.77(s,3H),3.50-3.30(m,1H),2.88(d,1H),2.72(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com