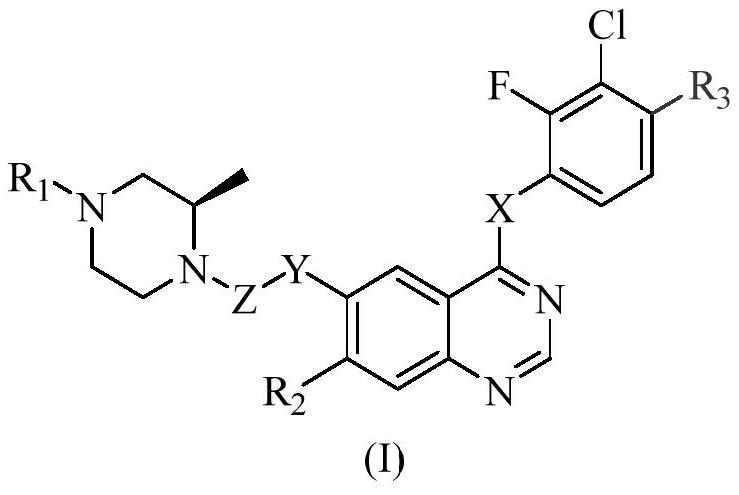
Polysubstituted quinazoline compound and application thereof
A compound, deuterated methyl technology, applied in the field of multi-substituted quinazoline compounds, can solve the problems of inability to reach tumor cells and poor curative effect, and achieve good pharmacodynamic performance, high inhibitory activity, and excellent brain barrier penetration performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1: 4-[(3-Chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl(R)-2-methyl-4-(cyclopropylmethyl ) piperazine-1-carboxylate
[0148]
[0149] Cyclopropylcarbaldehyde (47.24 mg, 0.67 mmol) was added to a solution of intermediate 6 (200 mg, 0.45 mmol) in dichloromethane (15 mL), and the reaction was stirred at room temperature for 0.5 h under nitrogen atmosphere. A mixture of sodium cyanoborohydride (113 mg, 1.8 mmol), acetic acid (2 mL) and methanol (10 mL) was added to the reaction system, stirred at room temperature for 2 h, and the reaction was completed by TLC detection. Water was added to the reaction system, the pH value was adjusted to 8.0-9.0 with saturated aqueous sodium bicarbonate solution, the layers were separated, extracted with dichloromethane, the organic layers were combined, dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure, and the mixture was subjected to silica gel column chromatography ( Dichloromethan...
Embodiment 2
[0150] Example 2: 4-[(3-Chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl(R)-2-methyl-4-(2-ethylbutane) yl)-piperazine-1-carboxylate
[0151]
[0152] The synthetic method refers to Example 1, wherein 2-ethylbutanal replaces cyclopropylcarbaldehyde.
[0153] MS-ESI(m / z):530.30[M+H] + ; 1 H-NMR (400MHz, DMSO-d 6 )δ: 9.73(s, 1H), 8.47(s, 1H), 8.22(s, 1H), 7.56~7.45(m, 2H), 7.33(s, 1H), 7.28(t, J=7.7Hz, 1H ), 4.41~4.20(m, 1H), 3.95(s, 3H), 3.86~3.64(m, 1H), 3.31~2.27(m, 1H), 2.85(d, J=11.2Hz, 1H), 2.73( d, J=11.4Hz, 1H), 2.17 (dd, J=12.3, 7.6Hz, 1H), 2.10 (dd, J=12.1, 6.6Hz, 2H), 2.00~1.92 (m, 1H), 1.48~1.43 (m,1H),1.40~1.28(m,7H),0.86(t,J=7.4Hz,6H).
Embodiment 3
[0154] Example 3: 4-[(3-Chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl(R)-2-methyl-4-(2-methylpentan yl)piperazine-1-carboxylate
[0155]
[0156] The synthetic method refers to Example 1, wherein 2-methylpentanal replaces cyclopropylcarbaldehyde.
[0157] MS-ESI(m / z):530.30[M+H] + ; 1 H NMR (400MHz, DMSO-d 6 )δ: 9.73(s, 1H), 8.47(s, 1H), 8.22(s, 1H), 7.54~7.46(m, 2H), 7.33(s, 1H), 7.28(t, J=7.7Hz, 1H ), 4.42~4.25(m, 1H), 3.95(s, 3H), 3.86~3.72(m, 1H), 3.28~3.15(m, 1H), 2.83(t, J=12.0Hz, 1H), 2.73( t,J=10.6Hz,1H),2.18~1.92(m,4H),1.67(s,1H),1.45~1.28(m,6H),1.13~1.01(m,1H),0.90~0.86(m, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com