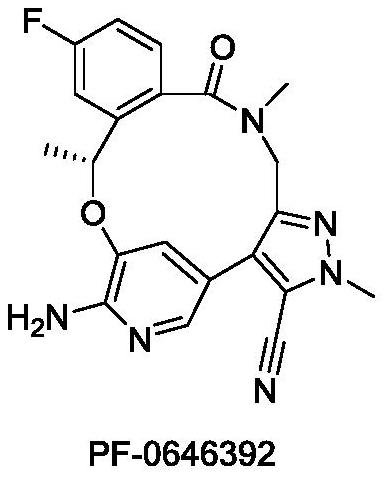
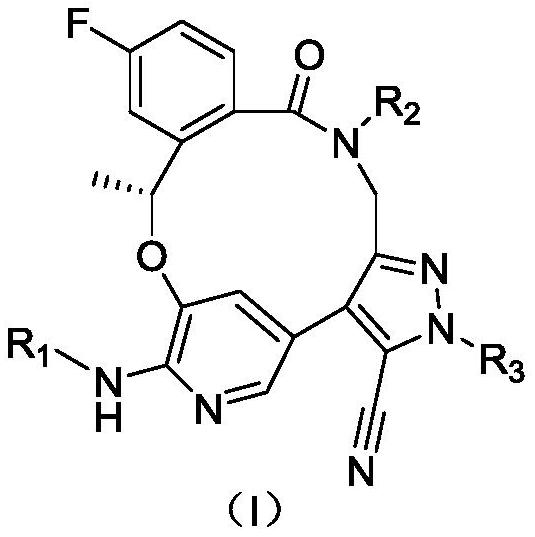
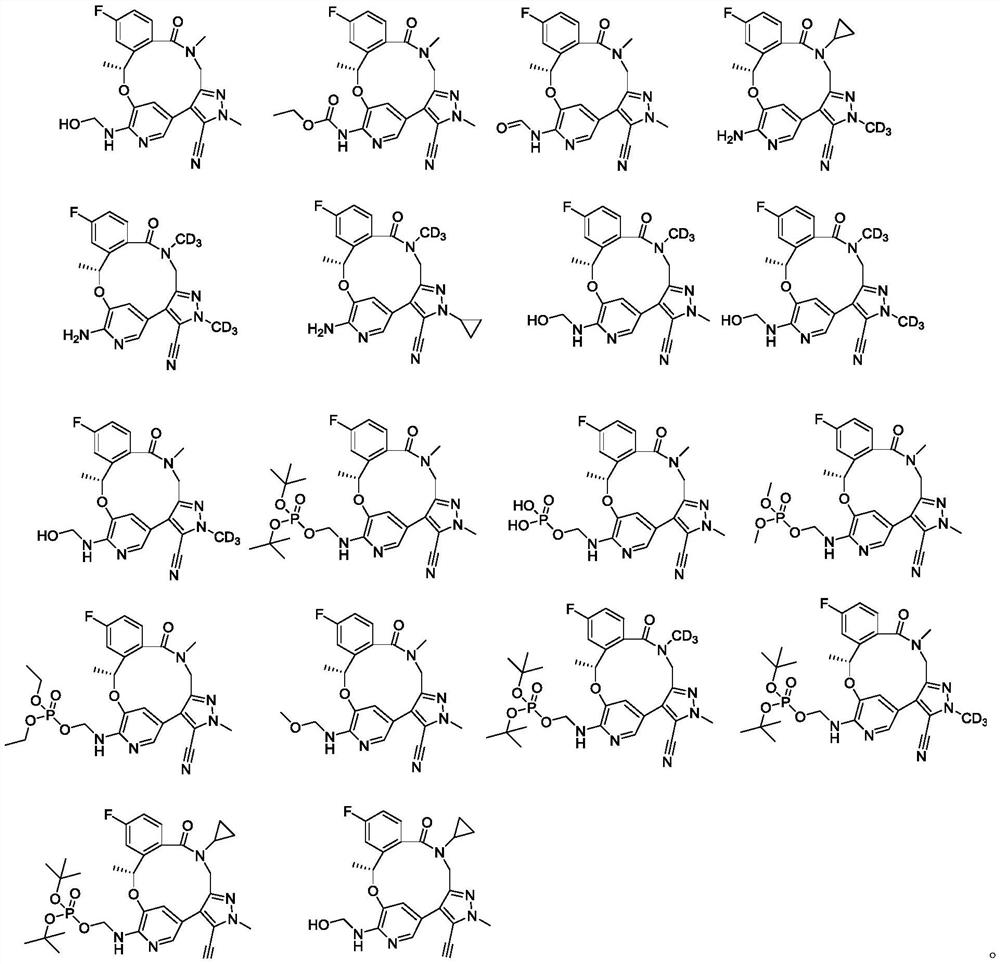
Benzoxadiazatetradecene derivatives and uses thereof
A technology of benzoxadiazatetradecene and its derivatives, which is applied in the field of benzoxadiazatetradecene derivatives, can solve the problems of easy drug resistance, large drug dosage, and toxicity Side effects and other problems, to achieve the effect of prolonging the drug's large metabolic half-life, excellent pharmacodynamic performance, and good inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Example 1: (10R)-7-Hydroxymethylamino-12-fluoro-2,10,16-trimethyl-5-oxo-10,15,16,17-tetrahydro-2H-8,4 -(Methylene bridge)-pyrazolo[4,3-h][2,5,11]benzoxadiazepinetetradecane-3-carbonitrile (Compound 1)
[0146]
[0147] Intermediate 18 (100 mg, 0.245 mmol) was suspended in water (1 mL), dichloromethane (1 mL), methanol (1 mL), and aqueous formalin (37%, 0.4 mL) and tetrabutylammonium fluoride (1 M were added) , 0.08mL). After overnight at room temperature, the reaction solution was extracted with dichloromethane, the organic phase was washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and purified by column to obtain 80 mg of solid with a yield of 74.7%.
[0148] ESI-MS m / z: 437.2[M+H] + .
[0149] l HNMR (400MHz, DMSO-d 6 )δ: 9.29(s, 1H), 7.61-7.58(m, 2H), 7.48-7.45(m, 1H), 7.20-7.16(m, 1H), 6.81(s, 1H), 6.39(d, J= 4.0Hz, 2H), 5.61(q, J=8.0Hz, 1H), 4.44(d, J=16.0Hz, 1H), 4.20(d, J=12.0Hz, 1H), 4.04(s, 3H), 2.99 (s,3H),1.68(d,J=8.0Hz,3H...
Embodiment 2
[0150]Example 2: (10R)-7-ethylcarbamate-12-fluoro-2,10,16-trimethyl-5-oxo-10,15,16,17-tetrahydro-2H-8,4 -(Methylene bridge)-pyrazolo[4,3-h][2,5,11]benzoxadiazepinetetradecane-3-carbonitrile (compound 2)
[0151]
[0152] Intermediate 18 (100 mg, 0.245 mmol) was dissolved in THF, DIPEA (38 mg, 0.294 mmol) was added, and ethyl chloroformate (26.5 mg, 0.245 mmol) was slowly added at 0 °C, and then stirred at room temperature overnight. Washed with water, extracted with ethyl acetate, the organic phase was dried and spin-dried, and purified by column to obtain 35 mg, yield: 29.7%.
[0153] ESI-MS m / z: 479.2[M+H] + .
[0154] l HNMR (400MHz, DMSO-d 6 )δ: 9.51(s, 1H), 7.98(s, 1H), 7.83-7.80(m, 1H), 7.49-7.47(m, 1H), 7.21-7.16(m, 2H), 5.66(q, J= 8.0Hz, 1H), 4.48(d, J=16.0Hz, 1H), 4.25(d, J=12.0Hz, 1H), 4.18(q, J=8.0Hz, 2H), 4.08(s, 3H), 3.00 (s,3H),1.68(d,J=8.0Hz,3H),1.28(t,J=8.0Hz,3H).
Embodiment 3
[0155] Example 3: (10R)-7-Aminocarboxy-12-fluoro-2,10,16-trimethyl-5-oxo-10,15,16,17-tetrahydro-2H-8,4- (Methylene bridge)-pyrazolo[4,3-h][2,5,11]benzoxadiazepinetetradecane-3-carbonitrile (compound 3)
[0156]
[0157] Under ice bath conditions, intermediate 18 (100 mg, 0.245 mmol) was slowly added to formic acid (113 mg, 2.45 mmol), acetic anhydride (37 mg, 0.367 mmol) was slowly added, and after the addition was completed, the mixture was stirred at room temperature overnight. The reaction solution was diluted with ethyl acetate, washed with water, dried, concentrated, and subjected to column chromatography to obtain 42 mg of solid, yield: 39.2%.
[0158] ESI-MS m / z: 435.2[M+H] + .
[0159] l HNMR (400MHz, DMSO-d 6 )δ: 10.42(d, J=12.0Hz, 1H), 9.23(s, 1H), 7.89(s, 1H), 7.81(d, J=8.0Hz, 1H), 7.50-7.46(m, 1H), 7.21-7.17(m, 2H), 5.73(q, J=8.0Hz, 1H), 4.48(d, J=16.0Hz, 1H), 4.23(d, J=12.0Hz, 1H), 4.08(s, 3H ),3.01(s,3H),1.72(d,J=8.0Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com