Application of polyiodobenzoic acid as cvb3 virus inhibitor
A technology of iodobenzoic acid and diiodobenzoic acid, which is applied in antiviral agents, active ingredients of salicylic acid, medical preparations containing active ingredients, etc., can solve the problems of unreported inhibitory activity and achieve enhanced cell survival High rate, high therapeutic index, good anti-CVB3 virus effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
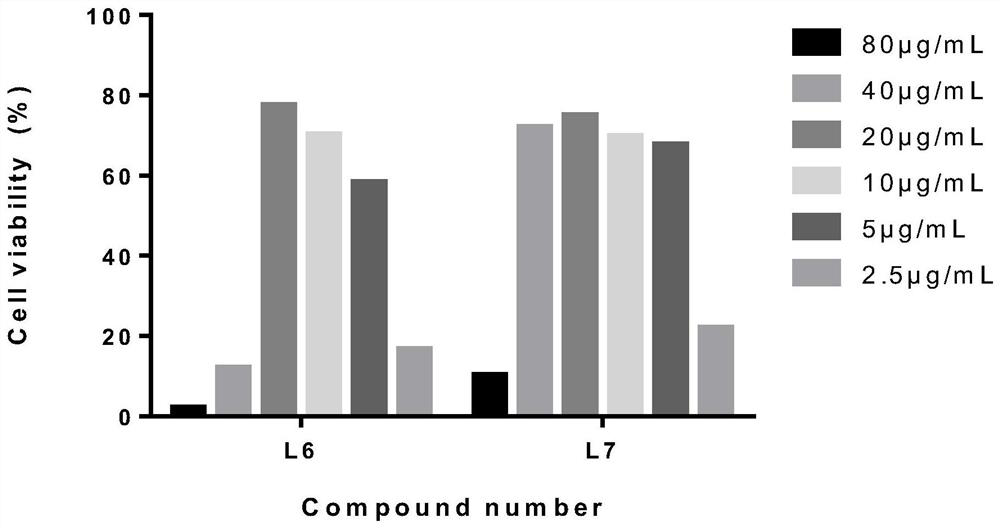
[0027] Embodiment 1: polyiodobenzoic acid L 6 , L 7 Toxicity to host Hep-2 cells
[0028] Hep-2 cells were plated in 96-well plates at 37°C, 5% CO 2 After the incubator grows a monolayer, discard the cell culture medium and add different concentrations of L 6 , L 7 After 48 hours, the cytotoxicity was recorded under a microscope, and the cell viability was measured by MTT method. The specific steps of the MTT method are as follows: add 30 μL of MTT (5 mg·mL -1 ), after incubation for 3-4 h, the supernatant was removed, and 50 μL of DMSO was added to dissolve the precipitate. Read the corresponding absorbance (OD) at 492 nm with a microplate reader 492 value).
[0029] SPSS 11.5 software was used to calculate the median toxic concentration (Medencyctoxic concentration, CC50) of the drug on the cells.
[0030] Cell viability=(average OD of drug group 492 Value / average OD of cell control group 492 value)×100%
Embodiment 2
[0031] Embodiment 2: polyiodobenzoic acid L 6 , L 7 Inhibitory activity against CVB3
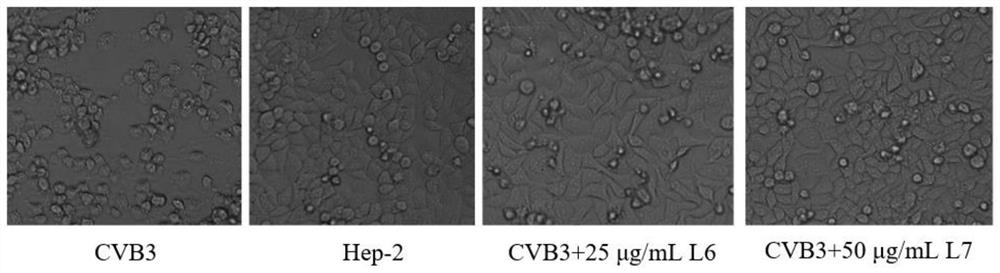
[0032] Hep-2 cells were plated in 96-well plates at 37°C, 5% CO 2 After the monolayer was grown in the incubator, discard the culture medium, infect the cells with 100TCID50 CVB3 virus solution for 1h, and add different concentrations (2.5μg / mL, 5μg / mL, 10μg / mL, 20μg / mL, 40μg / mL, 80μg / mL) of compound L 6 , L 7 (ribavirin as a positive control drug) to incubate the cells. After continuing to culture for about 48 hours, when about 90% of the CPE lesions appeared in the virus control wells, the cytopathic effect (CPE) was observed under a microscope. Observation and recording method of CPE: No cytopathic disease is marked as -, cytopathic disease of less than 25% is recorded as +, 25%-50% cytopathic disease is recorded as ++, 50%-75% cytopathic disease is recorded as +++, more than 75% Cytopathy was recorded as ++++.
[0033] After the observation of CPE, the inhibitory rate of drugs on...
Embodiment 3
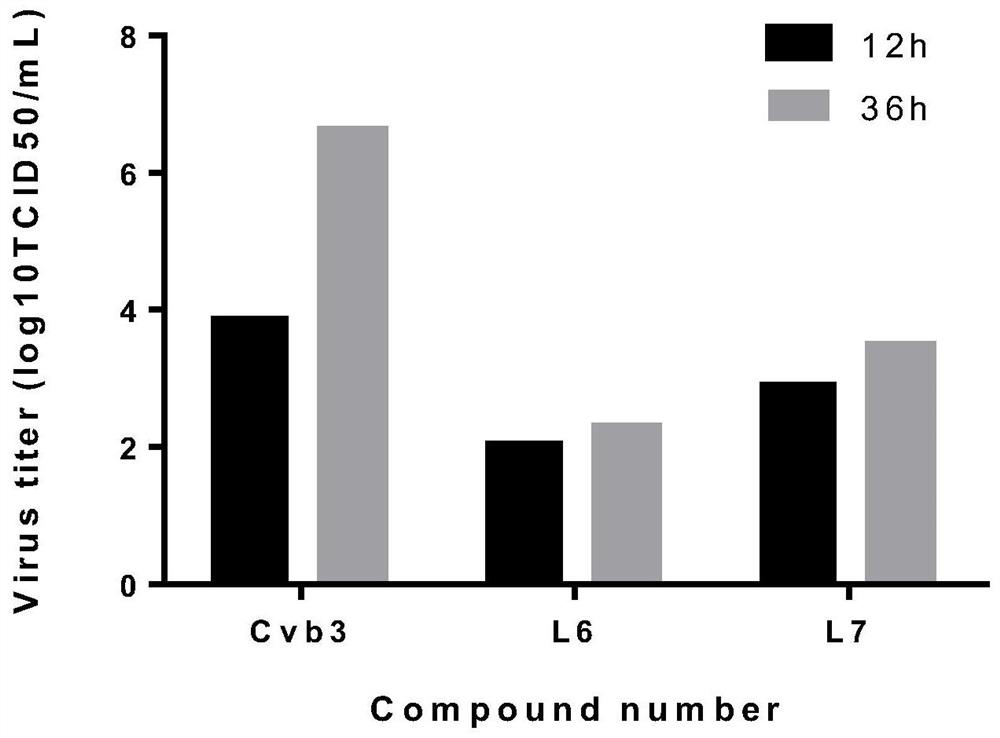
[0045] Embodiment 3: polyiodobenzoic acid L 6 , L 7 Inhibition of CVB3 Progeny Virus Yield
[0046] Hep-2 cells in the logarithmic growth phase were plated in 24-well plates, and 100TCID after the monolayer was overgrown 50 CVB3 infected cells, incubated at 37°C for 1.5h, removed the virus solution, washed three times with PBS, and added cell maintenance solution containing 25 μg / mL L6 and 50 μg / mL L7 respectively. Cells and supernatant culture fluid were collected at 12h and 36h respectively, and after three freeze-thaw lysis at -20°C and 37°C, TCID 50 Methods To determine the titer of CVB3 virus.
[0047] The result is as image 3 As shown, the CVB3 virus control group showed obvious virus titers at 12 hours after infection, and the virus titers rose rapidly by about 3.0log until 36 hours after infection. However, the virus titers of the 25 μg / mL L6 and 50 μg / mL7 treatment groups were lower than those of the virus control group under the same time conditions, and the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com