Pharmaceutical composition containing BPI-7711 and preparation method of pharmaceutical composition
A technology of BPI-7711 and composition, applied in the field of pharmaceutical preparations, can solve the problems of difficult industrial production and long-term storage of pharmaceuticals, increasing the risk of exceeding the limit of microbes of pharmaceuticals, and accelerating the degradation of pharmaceuticals, so as to avoid exposure to humidity and heat, Ensure stability and controllability, and ensure the effect of the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
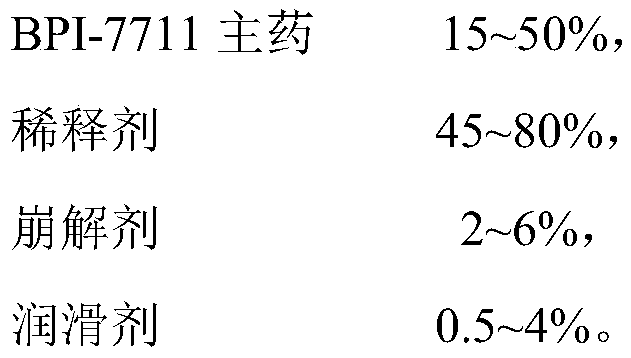
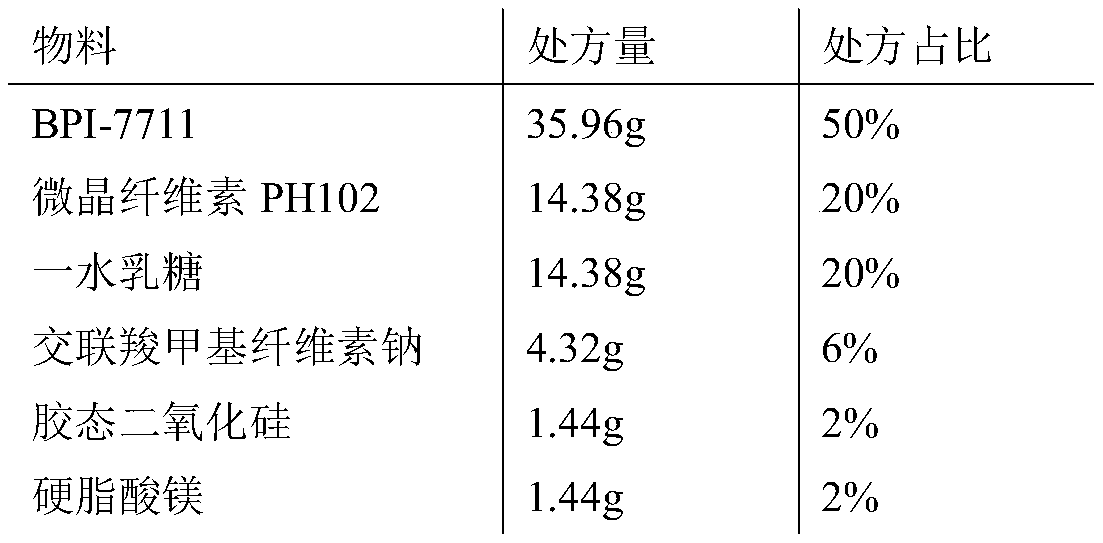
[0030] Calculated by making 1000 capsules, the components of the capsules are as follows:
[0031]
[0032] Among them: BPI-7711 particle size D 90 : 200μm, microcrystalline cellulose particle size pH102 D 90 : 204μm, lactose monohydrate particle size D 90 : 200 μm.
[0033] Prepare according to the following process:
[0034] a. Sieving: pass microcrystalline cellulose pH102, lactose monohydrate, and croscarmellose sodium through a 45-mesh sieve; use another 45-mesh sieve to pass through BPI-7711, and weigh the prescribed amount of BPI-7711 after sieving ;
[0035] b. Premixing: Put the sieved material into a 1L hopper mixer, set the mixer speed to 20 rpm, and mix for 24 minutes;
[0036] c. Mixing: Pass the colloidal silica through a 45-mesh sieve and add it to the mixer in step b, set the mixer speed to 20 rpm, and continue mixing for 14 minutes;
[0037] d. Lubrication: After passing the magnesium stearate through a 45-mesh sieve, add it to the c-step mixer, set t...
Embodiment 2
[0040] Calculated by making 1000 capsules, the components of the capsules are as follows:
[0041]
[0042] Among them: BPI-7711 particle size D 90 60μm, microcrystalline cellulose particle size D 90 : 100μm, lactose particle size D 90 : 105 μm.
[0043] Prepare according to the following process:
[0044] a. Sieving: pass microcrystalline cellulose, lactose monohydrate, and croscarmellose sodium through a 45-mesh sieve; use another 45-mesh sieve to pass through BPI-7711, and weigh the prescribed amount of BPI-7711 after sieving;
[0045] b. Premixing: put the sieved material into a 500L hopper mixer, set the mixer speed to 20 rpm, and mix for 24 minutes;
[0046] c. Mixing: Pass the colloidal silica through a 45-mesh sieve and add it to the mixer in step b, set the mixer speed to 20 rpm, and continue mixing for 14 minutes;
[0047] d. Lubrication: After passing the magnesium stearate through a 45-mesh sieve, add it to the c-step mixer, set the mixer speed to 20 rpm, an...
Embodiment 3
[0050] Calculated by making 1000 capsules, the components of the capsules are as follows:
[0051]
[0052]
[0053] Among them: BPI-7711 particle size D 90 : 120μm, microcrystalline cellulose pH102 particle size D 90 : 200μm, lactose particle size D 90 : 205 μm.
[0054] Prepare according to the following process:
[0055] a. Sieving: pass microcrystalline cellulose PH102, lactose monohydrate, and croscarmellose sodium through a 45-mesh sieve; use another 45-mesh sieve to pass through BPI-7711, and weigh the prescribed amount of BPI-7711 after sieving ;
[0056] b. Premixing: Put the sieved material into a 1L hopper mixer, set the mixer speed to 20 rpm, and mix for 24 minutes;
[0057] c. Mixing: Pass the colloidal silica through a 45-mesh sieve and add it to the mixer in step b, set the mixer speed to 20 rpm, and continue mixing for 14 minutes;
[0058] d. Lubrication: After passing the magnesium stearate through a 45-mesh sieve, add it to the c-step mixer, set t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com