Preparation method for ARV-110 intermediate
A technology of oxo and ethoxy, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of many by-products, complex reactions, and difficulties in scale-up production, and achieve the effects of mild reaction conditions, simple operation, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
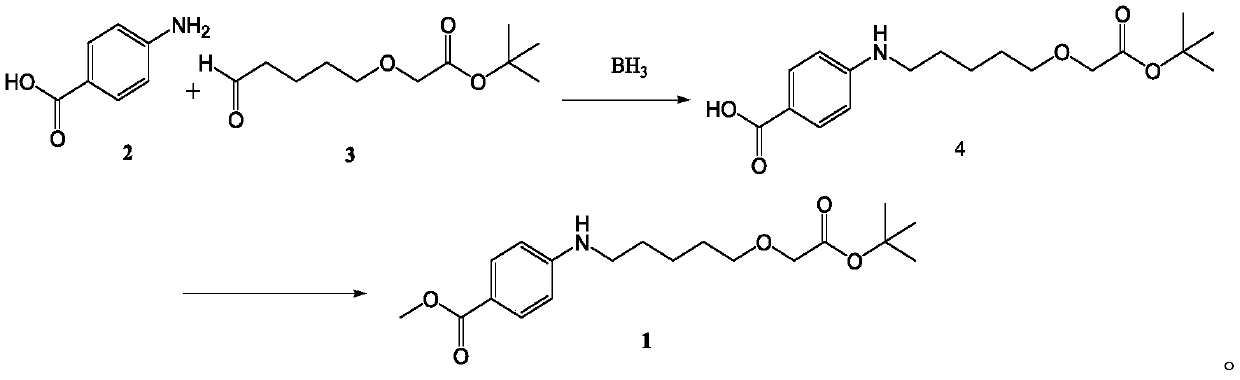
[0031] The invention provides a kind of preparation method of 4-((5-(2-(tert-butoxy)-2-oxoethoxy) pentyl) amino) methyl benzoate, described method comprises following two step:
[0032] (1) Compound p-aminobenzoic acid 2 and aliphatic aldehyde 3 in BH 3 Under the action of the compound 4-((5-(2-(tert-butoxy)-2-oxoethoxy)phenyl)pentylamino)benzoic acid 4;
[0033] (2) compound 4-((5-(2-(tert-butoxy)-2-oxoethoxy) phenyl) amino) benzoic acid 4 is esterified in the system of halide / alkali, The compound methyl 4-((5-(2-(tert-butoxy)-2-oxoethoxy)pentyl)amino)benzoate 1 was obtained.
[0034] The reaction synthesis route is:
[0035]
[0036] In a preferred embodiment, in step (1), the reaction solvent is a common organic solvent in the art, preferably, the reaction solvent is selected from tetrahydrofuran, 2-methyltetrahydrofuran, ethylene glycol dimethyl ether, methanol, ethanol , acetonitrile, ethyl acetate, isopropyl acetate and one or more of methyl tert-butyl ether.
[...
Embodiment 1
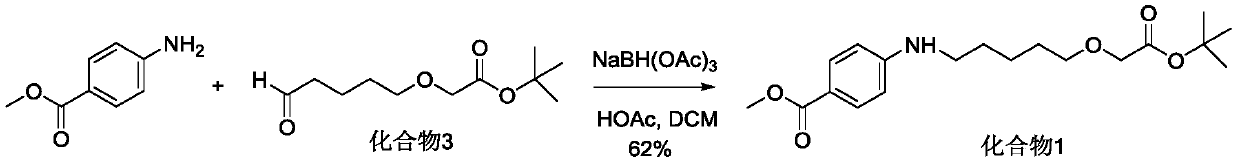
[0047] Preparation of 4-((5-(2-(tert-butoxy)-2-oxoethoxy)phenyl)amino)benzoic acid
[0048]
[0049] P-aminobenzoic acid (100g, 0.73moL, purchased from Biide Pharmaceuticals) and BH 3Tetrahydrofuran complex solution (800 mL, 1.0 M, 0.80 moL, purchased from Anaiji Chemical) was added to tetrahydrofuran (2.5 L). After the addition, stir at room temperature for 30 minutes, and then add aliphatic aldehyde (174.0g, 0.80moL, self-made according to the method provided on page 169 of CN201680014250.8). Stir at room temperature overnight, spot plate detection reaction is complete, add water to quench the reaction. The solvent was evaporated under reduced pressure, water (2.0 L) was added to the residue, and the pH value was adjusted to 12 with 4N NaOH solution. Add ethyl acetate (500mL) and separate the layers. The aqueous phase was collected, and the pH value of the aqueous phase was adjusted back to about 3 with concentrated hydrochloric acid. Extract with ethyl acetate (2 x 1...
Embodiment 2
[0051] Preparation of 4-((5-(2-(tert-butoxy)-2-oxoethoxy)phenyl)amino)benzoic acid
[0052]
[0053] Except that the reaction solvent was replaced by 2-methyltetrahydrofuran, the operation of Example 2 was the same as that of Example 1, and the yield was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com