Niclosamide ethanolamine salt-hydroxypropyl-beta-cyclodextrin inclusion compound and preparation thereof
A technology for niclosamide ethanolamine salt and ethanolamine salt hydroxypropyl, which is applied in the field of niclosamide ethanolamine salt-hydroxypropyl-β-cyclodextrin and its preparation, can solve the problems of less research on injection preparations and the like , to achieve the effect of strong solubilization, small local irritation and low hemolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] One, the preparation method of clathrate
[0022] The invention provides a preparation method of niclosamide ethanolamine salt-hydroxypropyl-β-cyclodextrin inclusion compound, which comprises the following steps, and all the steps of the invention are completed under light-proof conditions.
experiment example 1
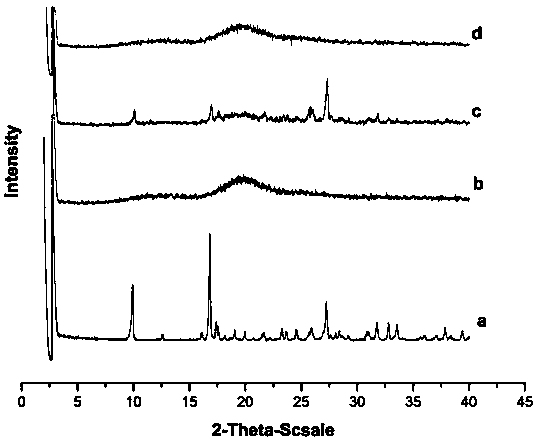
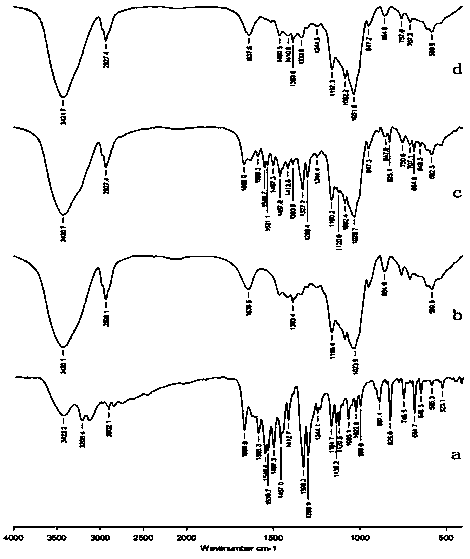
[0023] Experimental Example 1 Quality Evaluation of NHC Inclusion Compounds Prepared by Different Preparation Methods
[0024] Grinding method (Kneading method)
[0025] Weigh about 0.25g of NES powder and 10g of HP-β-CD, mix them in a mortar, add appropriate amount of distilled water and grind until thick slurry (about 2h). Dry at 40°C, pulverize, and pass through a 80-mesh sieve to obtain a yellow powder product.
[0026] Freeze-drying (Co-lyophilized)
[0027] Weigh 0.25 g of NES powder, and measure 25 mL of HP-β-CD solution with a mass concentration of about 400 g / L. Heat the water temperature to 60°C under magnetic stirring and slowly put NES into the aqueous solution of HP-β-CD, keep stirring at a constant temperature for 2 hours to obtain a clear solution, pre-freeze in a -80°C refrigerator for 18 hours, and freeze-dry in a vacuum freeze dryer for 24 hours , the dry matter was pulverized and passed through a 80-mesh sieve to obtain a yellow powder product.
[0028] ...
experiment example 2
[0034] Experimental Example 2 Quality Evaluation of NHC Inclusion Compounds Prepared by Different Concentrations of HP-β-CD Solutions
[0035] Prepare 10mL of cyclodextrin solutions with concentrations of 200g / L, 250g / L, 300g / L, 350g / L, 400g / L, and 500g / L, respectively, and put them into NES according to the ratio of substances of 11:1. Under the condition of water bath at 45°C, NES was slowly added into the HP-β-CD aqueous solution while stirring, and the constant temperature stirring was continued for 2 hours to obtain a clear solution. The inclusion ratios were calculated respectively, and the results are shown in Table 2.
[0036] Table 2 Quality evaluation of NHC clathrates prepared by different concentrations of HP-β-CD solutions
[0037]
[0038] Experimental results: The NHC clathrate prepared by using the concentration of HP-β-CD (300g / L) of the present invention has a high clathrate rate and good clathrate effect. The results show that only the NHC clathrate prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com