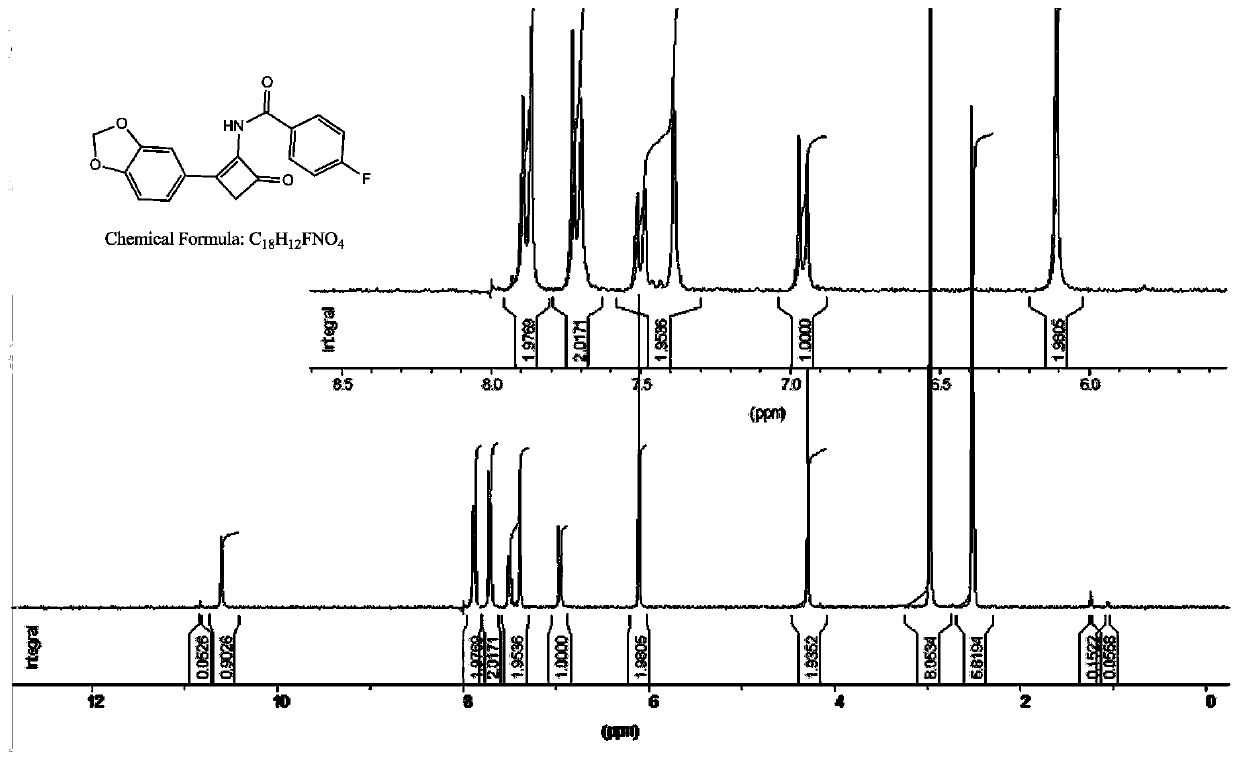
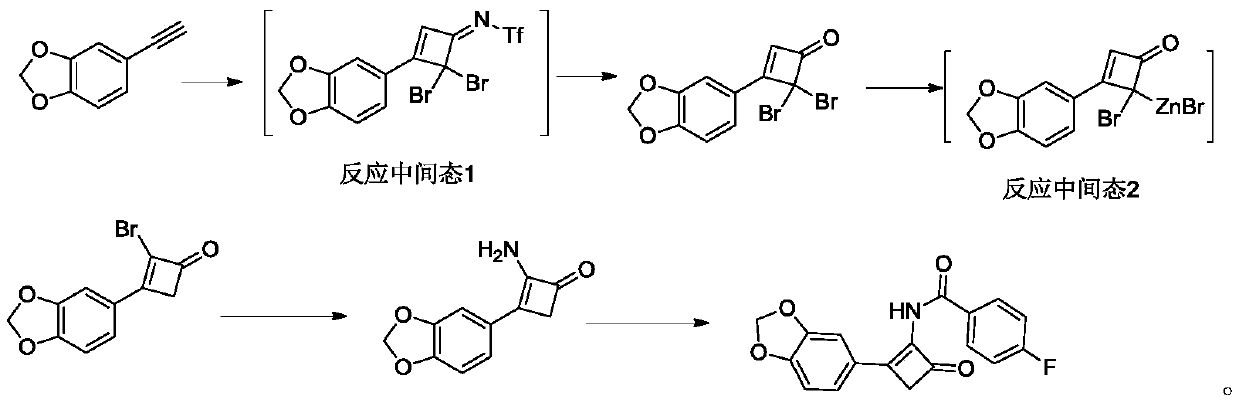
Cyclobutenone compound with antioxidant effect and preparation method thereof
A technology of anti-oxidative effect and cyclobutenone, which is applied in the fields of organic chemistry, anti-toxic agent, drug combination, etc., can solve the problems of limited regional selectivity of reaction raw materials, poor synthesis of reaction substrates, etc., and achieve good anti-oxidative effect, The effect of novel structure and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025]In a reaction flask with a water separator, under the protection of nitrogen, add (triphenylphosphine) gold(I) chloride 1g, trifluoromethanesulfonic anhydride 34g and 1,8-bisdimethylaminonaphthalene 21g Add 200mL of toluene, slowly heat to reflux, remove the water contained in the reaction system through the water separator, then cool down to room temperature, add 20g of dibromoacetonitrile, heat to reflux again, further remove the water in the reaction system, and cool down to room temperature again , under the protection of nitrogen, quickly add 15 g of 5-alkynyl-benzo[d][1,3]dioxole, and slowly increase the temperature to 70 °C under the protection of nitrogen, and react for about 3 hours. Add 500mL of water to the solution, continue to heat to reflux, continue to react for 5h, adjust the pH to 4-5 with 1N hydrochloric acid solution, extract 100mL of the extraction reaction solution with ethyl acetate several times, combine the organic layers with 100mL of ...
Embodiment 2
[0027]
[0028] In a reaction flask with a water separator, under the protection of nitrogen, put (triphenylphosphine) gold(I) chloride 0.5g, trifluoromethanesulfonic anhydride 34g and 1,8-bisdimethylaminonaphthalene 21g Add it into 200mL of toluene, slowly heat to reflux, remove the water contained in the reaction system through a water separator, then cool down to room temperature, add 20g of dibromoacetonitrile, heat to reflux again, further remove the water in the reaction system, and drop to room temperature again. At room temperature, under the protection of nitrogen, quickly add 15 g of 5-alkynyl-benzo[d][1,3]dioxole, and slowly increase the temperature to 70°C under the protection of nitrogen, and react for about 3 hours. Add 500mL of water to the reaction solution, continue heating to reflux, continue to react for 5h, adjust the pH to 4-5 with 1N hydrochloric acid solution, extract 100mL of the reaction solution with ethyl acetate for several times, combine the orga...
Embodiment 3
[0030]
[0031] In a reaction flask with a water separator, under the protection of nitrogen, put (triphenylphosphine) gold(I) chloride 0.5g, trifluoromethanesulfonic anhydride 34g and 1,8-diazabicyclodeca Add 15 g of one-carbon-7-ene into 200 mL of toluene, slowly heat to reflux, remove the water contained in the reaction system through a water separator, then cool down to room temperature, add 20 g of dibromoacetonitrile, heat to reflux again, and further remove the reaction system The water in the solution was lowered to room temperature again, and under the protection of nitrogen, 15 g of 5-alkynyl-benzo[d][1,3]dioxole was added quickly, and the temperature was slowly raised to 70 under the protection of nitrogen. ℃, react for about 3 hours, add 500 mL of water to the reaction solution, continue to heat to reflux, continue to react for 5 hours, adjust the pH to 4-5 with 1N hydrochloric acid solution, extract 100 mL of the reaction solution with ethyl acetate for several ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com