Triple inactivated vaccine for haemophilus parasuis diseases, streptococcosis suis diseases and pasteurella multocida diseases of pigs and preparation method of triple inactivated vaccine
A technology for Haemophilus suis disease and Haemophilus suis, applied in the field of veterinary biological products, can solve the problems of increasing the morbidity and mortality of respiratory diseases in pigs, affecting the safety of animal body and human consumption, and damaging the antigenicity of vaccines. , to achieve the effect of improving antigen utilization, convenient and fast immunization, and improving targeted delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
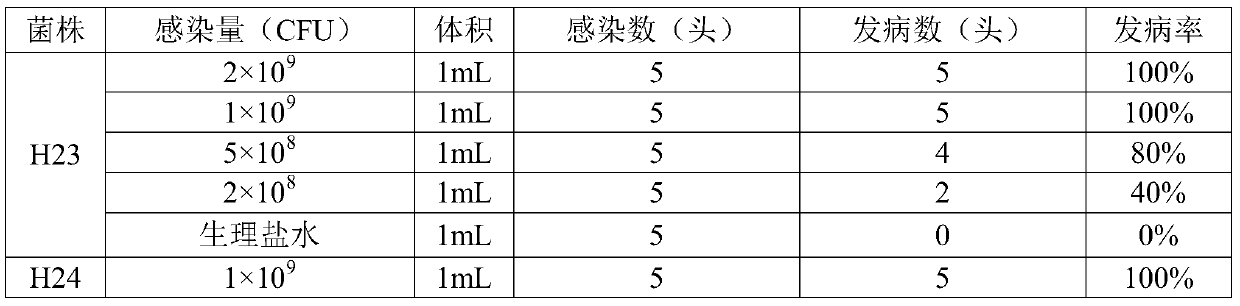
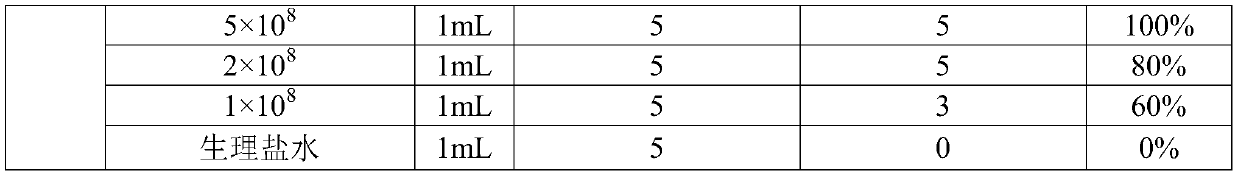
[0032] The acquisition of embodiment 1 bacterial strain antigen solution
[0033] 1. Isolation, identification and virulence test of Haemophilus parasuis H23 and H24 strains
[0034] 1.1 Isolation and identification of Haemophilus parasuis strains H23 and H24
[0035] Animals: From 2016 to 2018, fresh diseased pigs with clinical manifestations such as panting, pleurisy, peritonitis, pericarditis, arthritis or encephalitis were collected from South China. They were nursery piglets 1-2 weeks after weaning.
[0036] Method: Aseptically collect the lungs, heart blood, and joint fluid of diseased pigs, inoculate them in TSA medium (containing 5% bovine serum, 0.002% NAD, and 100 μg / mL lincomycin) respectively, and culture them in a 37°C incubator for 24-48 After hours, observe the shape of bacterial colonies, select suspicious colonies for pure culture, and carry out 16S rRNA identification after expanding the culture of suspicious strains. The primer sequences are as follows:
...
Embodiment 2
[0090] Embodiment 2 Preparation of a triple inactivated vaccine of Haemophilus parasuis, Streptococcus suis and Pasteurellosis multocida
[0091] 1. Seed Preparation for Production
[0092] Primary seed propagation: Freeze-dried Haemophilus parasuis serotype 4 H23 strain (GDMCC No: 60722, storage place: Guangdong Microbial Culture Collection Center), Haemophilus parasuis serotype 5 H24 strain (GDMCC No: 60723, preservation place: Guangdong Microbial Culture Collection Center) inoculated in TSA containing 5% calf serum and 0.002% NAD, freeze-dried Streptococcus suis serotype 2 S23 strain (GDMCC No: 60724, preservation place: Guangdong Provincial Microbial Culture Collection Center) inoculated in TSA containing 5% calf serum, and Pasteurella multocida capsular type A P13 strain (GDMCC No: 60720, preservation place: Guangdong Provincial Microbiological Culture Collection Center ), Pasteurella multocida capsular type B P11 strain (GDMCC No: 60721, storage place: Guangdong Microbi...
Embodiment 3 3
[0108] The sterility test and safety test of embodiment 3 triple inactivated vaccines
[0109] Materials: Vaccine 1 and Vaccine 2. Vaccine 2 was prepared by inactivating each bacterial solution according to two inactivation methods respectively, and prepared according to the components of vaccine 2, and the preparation method was the same as that in Example 2.
[0110] The first inactivation method uses β-propiolactone solution as an inactivating agent to inactivate each bacterial liquid. The specific method is: slowly add β-propiolactone solution according to 0.1%-0.15% of the total bacterial liquid, and heat it at 4°C. Inactivate for 72 hours, stir several times during the period, and then hydrolyze the inactivator in a water bath at 37°C for 2 hours;
[0111] The second inactivation method is to use formaldehyde solution as an inactivator to inactivate each bacterial solution. The specific method is: slowly add formaldehyde solution according to 0.4% of the total bacterial...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com