Post-treatment method of working solution for producing hydrogen peroxide by using anthraquinone method
A hydrogen peroxide, working fluid technology, applied in chemical instruments and methods, peroxide/peroxyhydrate/peroxyacid/superoxide/ozone, inorganic chemistry, etc. Deterioration of device operating conditions, inability to meet post-processing requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
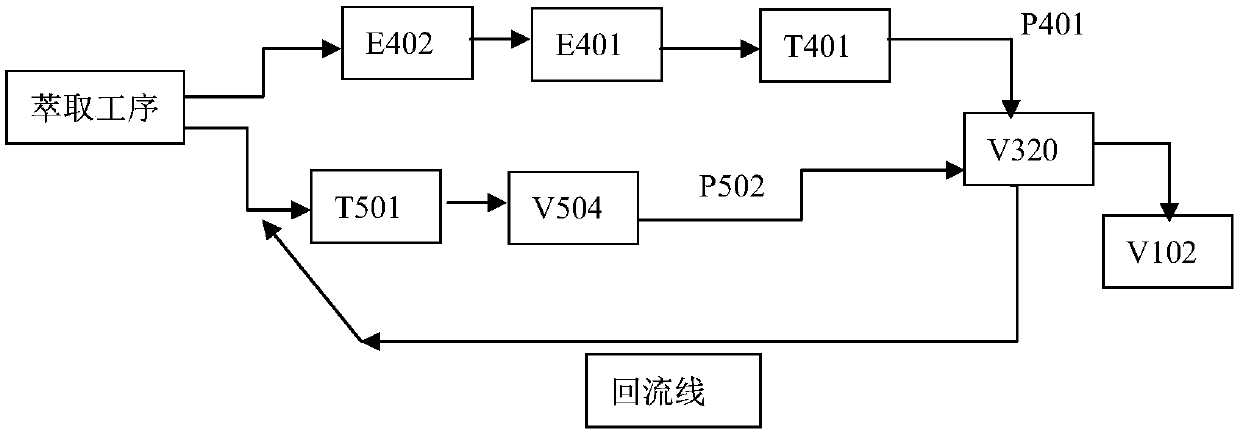
[0061] The raffinate in system A is preheated to 55°C through the heat exchanger (E402), then heated to 85°C through the vacuum feed heater (E401), and then dehydrated and dried in the vacuum drying tower (T401) , the pressure of the vacuum drying tower is 10.5KPa.
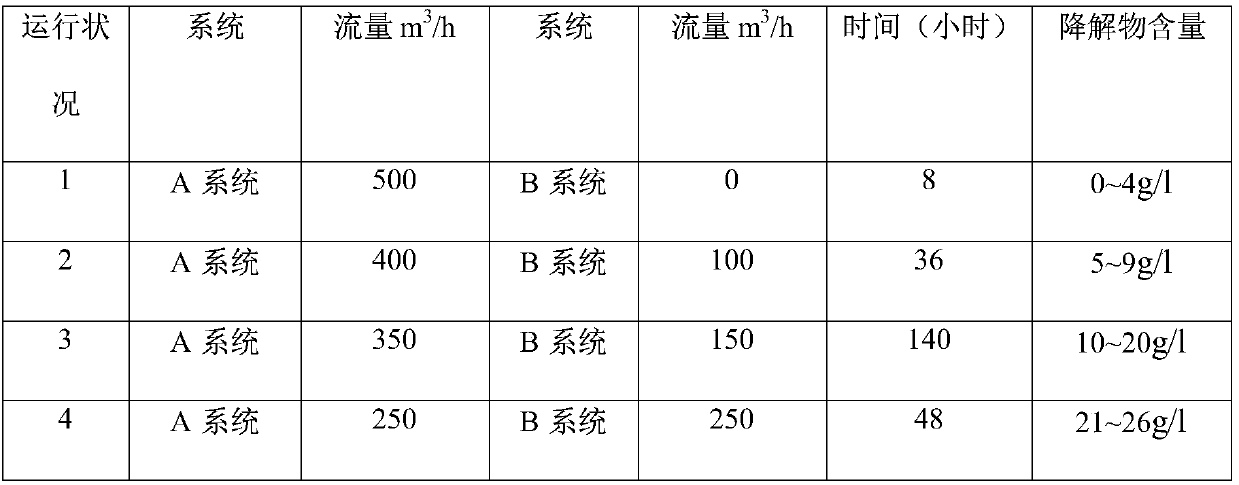
[0062] The flow distribution is shown in Table 1:
[0063] Table 1 Example 1 flow distribution table
[0064]
[0065] During the 232 hours of operation, the hydrogen efficiency is 7.0-7.5g / l, and the content of degradation products in the working fluid system is gradually and stably controlled between 11-18g / l. The flow rate of system A is 350m for three consecutive months. 3 / h, the flow rate of system B is 150m 3 Under the operation mode of / h, the content of degradation products in the working fluid system is still stably controlled between 11-18g / l, the raffinate index is controlled below 0.15g / l, and the total anthraquinone in the working fluid system is kept between 125-135g / l During the period, the p...
Embodiment 2
[0067] The raffinate in system A is preheated to 55°C through the heat exchanger (E402), then heated to 85°C through the vacuum feed heater (E401), and then dehydrated and dried in the vacuum drying tower (T401) , the pressure of the vacuum drying tower is 11KPa.
[0068] The flow distribution is shown in Table 2:
[0069] Table 2 Example 2 flow distribution table
[0070] operating status system Flow m 3 / h
[0071] During the 232 hours of operation, the hydrogen efficiency is 7.0-7.5g / l, and the content of degradation products in the working fluid system is gradually controlled between 10-15g / l, and the flow rate of system A is 322m for three consecutive months. 3 / h, the flow rate of system B is 72m 3 Under the operation mode of / h, the content of degradation products in the working fluid system is still stably controlled between 10-15g / l, the raffinate index is controlled below 0.15g / l, and the total anthraquinone in the working fluid system is kept betwe...
Embodiment 3
[0073] The raffinate in system A is preheated to 55°C through the heat exchanger (E402), then heated to 85°C through the vacuum feed heater (E401), and then dehydrated and dried in the vacuum drying tower (T401) , the pressure of the vacuum drying tower is 11.5KPa.
[0074] The flow distribution is shown in Table 3:
[0075] Table 3 Example 3 traffic distribution table
[0076] operating status system Flow m 3 / h
[0077] During the 232 hours of operation, the hydrogen efficiency is 7.0-7.3g / l, and the content of degradation products in the working fluid system is gradually controlled between 10-16g / l, and the flow rate of system A is 348m for three consecutive months. 3 / h, the flow rate of system B is 150m 3 Under the operation mode of / h, the content of degradation products in the working fluid system is still stably controlled between 10-16g / l, the raffinate index is controlled below 0.15g / l, and the total anthraquinone in the working fluid system is kept...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com