Indolylhydrazone derivative as well as preparation method and application thereof to prevention and control of plant viruses, sterilization and disinsection
A technology of indolehydrazone and derivatives, applied in the field of pesticides, to achieve excellent anti-plant virus activity and good anti-tobacco mosaic virus effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
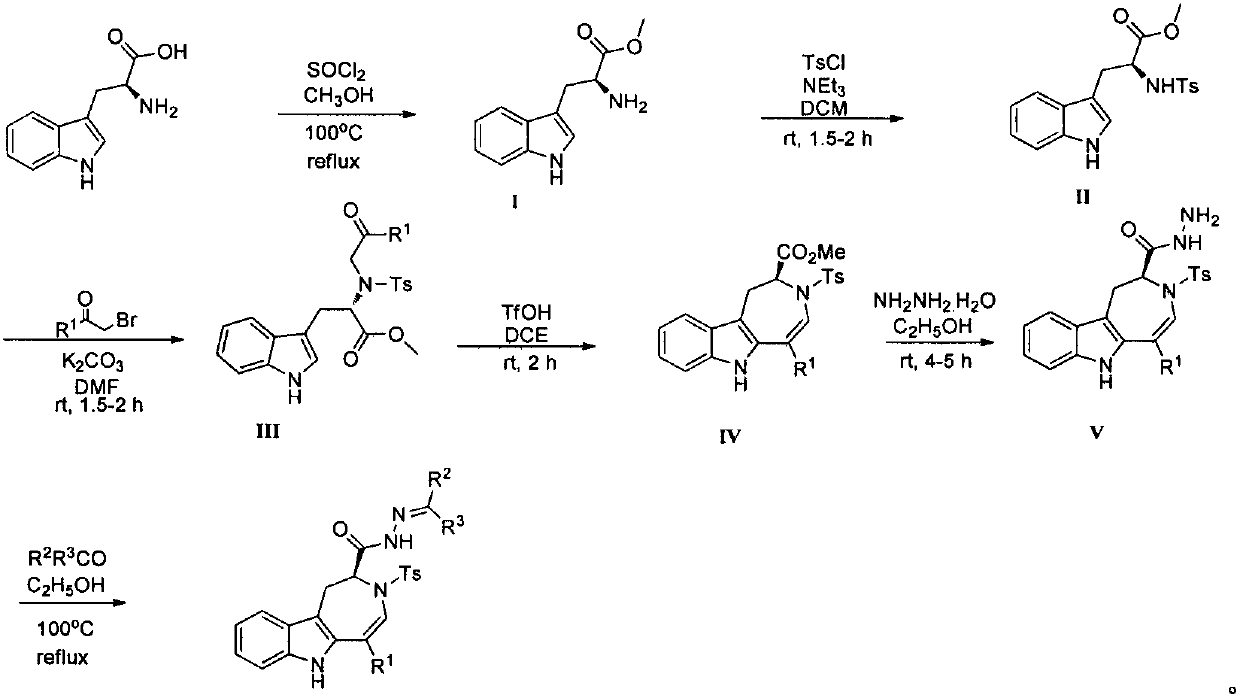
[0059] The present invention provides a kind of above-mentioned tetrahydroazepine The preparation method of [4,5-b] indolehydrazone derivatives, the method comprises: first, L-tryptophan in SOCl 2 Catalyzed esterification reaction with methanol to generate L-tryptophan methyl ester (I), L-tryptophan methyl ester under triethylamine alkaline conditions on the Ts protecting group to form II, II respectively with α-bromobenzene Ethanone undergoes nucleophilic substitution to generate III, and then undergoes dehydration condensation and ring-closing rearrangement under the catalysis of trifluoromethanesulfonic acid to generate the corresponding IV, IV undergoes hydrazine hydrolysis to generate the corresponding hydrazide V, and the intermediate V is aldehyde-condensed to obtain the general Tetrahydroazepine And [4,5-b] indolehydrazone compound;
[0060]
[0061] Among them, R 1 , R 2 , R 3 As described above, the present invention will not be repeated here.
[0062] The...
Embodiment 1
[0081] Embodiment 1: Tetrahydroazepine Synthesis of [4,5-b]indolehydrazone Derivative VI-1
[0082]
[0083] L-Tryptophan methyl ester (I)
[0084] 10.31 g (50 mmol) of L-tryptophan and 150 mL of anhydrous methanol were added to a 250 mL round bottom flask, and 10 mL of thionyl chloride was slowly added in an ice-water bath. After dropping, the solution was clarified and heated to reflux for 5 hours. After the reaction was complete as detected by TLC, the solvent was removed, washed with saturated sodium carbonate solution, extracted with ethyl acetate (50mL×3), washed with saturated brine three times, dried over anhydrous sodium sulfate, and suction filtered. Rotary evaporation gave 10.51 g of a brown solid, with a yield of 96%. Melting point: 90-91°C. 1 H NMR (400MHz, CDCl 3 )δ8.65 (s, 1H, Ar-NH), 7.60 (d, J=8.0Hz, 1H, Ar-H), 7.29 (d, J=8.0Hz, 1H, Ar-H), 7.16(t, J=7.2Hz, 1H, Ar-H), 7.10(t, J=7.2Hz, 1H, Ar-H), 6.95(d, J=2.4Hz, 1H, C=CH), 3.84(dd, J= 8.0, 4.8Hz, 1H, ...
Embodiment 2
[0169] Embodiment 2: the assay of anti-tobacco mosaic virus activity, assay procedure is as follows:
[0170] 1. Virus purification and concentration determination:
[0171] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0172] 2. Compound solution preparation:
[0173] After weighing, the original drug was dissolved in DMF to prepare 1×10 5 μg / mL mother solution, and then diluted with 1‰ Tween 80 aqueous solution to the required concentration; Ningnanmycin preparation was directly diluted with water.
[0174] 3. In vitro effect:
[0175] Rub inoculation of leaves of Shanxi tobacco at the right age, rinse with running water, the virus concentration is 10 μg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com